-
Name
Diethyl malonate
- EINECS 203-305-9
- CAS No. 105-53-3
- Article Data249
- CAS DataBase
- Density 1.06 g/cm3
- Solubility Miscible with ethyl alcohol, ether, chloroform and benzene. Slightly miscible with water.
- Melting Point -50 °C
- Formula C7H12O4
- Boiling Point 199.3 °C at 760 mmHg
- Molecular Weight 160.17
- Flash Point 100 °C
- Transport Information
- Appearance colourless liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Diethyl propanedioate;Propanedioic acid, diethyl ester;Carbethoxyacetic ester;Dicarbethoxymethane;Methanedicarboxylic acid, diethyl ester;Malonic ester;Malonic acid, diethyl ester;Ethyl Malonate;Propanedioic acid, 1,3-diethyl ester;
- PSA 52.60000
- LogP 0.50270
Synthetic route
-
-
130217-49-1
4-[(3-ethoxy-1,3-dioxopropyl)amino]-benzoic acid

-
A
-
10256-16-3
N,N'-di-4-carboxyanilide of malonic acid

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 98% B n/a |
-
-
101646-18-8
2-carboxymalonanilic acid ethyl ester

-
A
-
77317-57-8
N,N'-di-2-carboxyanilide of malonic acid

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 98% B n/a |

-
-
201230-82-2
carbon monoxide

-
A
-
61091-28-9, 15226-74-1, 61117-58-6
dicobalt octacarbonyl

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| With ethanol In hexane High Pressure; soln. of Co2(CO)8 and EtOH placed in autoclave; pressurized (room temp.,50 bar CO); shaken (60 min); IR; gas chromy.; | A 98% B 95% |

-
-
4270-39-7
4-ethoxymalonanilic acid ethyl ester

-
A
-
4270-37-5
N,N'-di-4-ethoxyanilide of malonic acid

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 97% B n/a |

| Conditions | Yield |
|---|---|
| With ethanol In dichloromethane High Pressure; soln. of cobalt complex and anhyd. ethanol pressurized with CO in autoclave at 25°C and 50 bar for 24 h; IR; | A 97% B 66% |

-
-
90475-72-2
2-methoxymalonanilic acid ethyl ester

-
A
-
7056-72-6
N1,N3-bis(2-methoxyphenyl)malonamide

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 96% B n/a |
-
-
43038-58-0
2-hydroxy-4-nitromalonanilic acid ethyl ester

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 96% B n/a |

| Conditions | Yield |
|---|---|
| With bismuth(III) oxide; N-ethyl-N,N-diisopropylamine; para-thiocresol In dichloromethane for 1h; Irradiation; | 96% |
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In 2,2,2-trifluoroethanol at 20℃; for 4h; UV-irradiation; Sealed tube; Green chemistry; | 91% |
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; N-ethyl-N,N-diisopropylamine; 4,7-bis(thiophen-2-yl)-2,1,3-benzothiadiazole In N,N-dimethyl-formamide for 2.5h; Inert atmosphere; Irradiation; | 87% |
-
-
159657-36-0
4-carboethoxymalonanilic acid ethyl ester

-
A
-
19288-86-9
N,N'-di-4-carboethoxyanilide of malonic acid

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 96% B n/a |

-
-
201230-82-2
carbon monoxide

-
A
-
14243-08-4
tricarbonyldi(triphenylphosphine)cobalt(1+) tetracarbonylcobaltate(1-)

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| With ethanol In dichloromethane High Pressure; soln. of cobalt complex and anhyd. ethanol pressurized with CO in autoclave at 25°C and 50 bar for 24 h; IR; | A n/a B 96% |

-
-
685-87-0
Diethyl 2-bromomalonate

-
A
-
632-56-4
tetraethyl ethane-1,1,2,2-tetracarboxylate

-
B
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; electrochemical reaction: -1,0 V, 0,1 mol/dm3 Et4NClO4, mercury pool cathode; | A 95% B 5% |
| With potassium hydrogenphosphate trihydrate; 2,6-di-tert-butyl-4-methyl-phenol In ethanol at 20℃; for 1h; Mechanism; Irradiation; Inert atmosphere; Green chemistry; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; | A 71% B 95% |

-
-
1071-46-1
hydrogen ethyl malonate

-
-
541-41-3
chloroformic acid ethyl ester

-
A
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 4℃; for 0.5h; | A 95% B n/a |


| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol | 95% |
Diethyl malonate Specification
The CAS registry number of Diethyl malonate is 105-53-3. The IUPAC name is diethyl propanedioate. In addition, the molecular formula is C7H12O4 and the molecular weight is 160.17. It is also called propanedioic acid, diethyl ester. What's more, it is the diethyl ester of malonic acid. And it is a colourless liquid with an apple-like odour. Besides, it can be used in perfumes. It is also used to synthesize other compounds such as barbiturates and artificial flavourings.
Physical properties about this chemical are: (1)ACD/LogP: 0.70; (2)ACD/LogD (pH 5.5): 0.7; (3)ACD/LogD (pH 7.4): 0.7; (4)ACD/BCF (pH 5.5): 2.02; (5)ACD/BCF (pH 7.4): 2.02; (6)ACD/KOC (pH 5.5): 57.48; (7)ACD/KOC (pH 7.4): 57.48; (8)#H bond acceptors: 4; (9)#Freely Rotating Bonds: 6; (10)Polar Surface Area: 52.6 Å2; (11)Index of Refraction: 1.417; (12)Molar Refractivity: 38.02 cm3; (13)Molar Volume: 151 cm3; (14)Polarizability: 15.07 ×10-24cm3; (15)Surface Tension: 32.3 dyne/cm; (16)Density: 1.06 g/cm3; (17)Flash Point: 100 °C; (18)Enthalpy of Vaporization: 43.55 kJ/mol; (19)Boiling Point: 199.3 °C at 760 mmHg; (20)Vapour Pressure: 0.344 mmHg at 25°C.
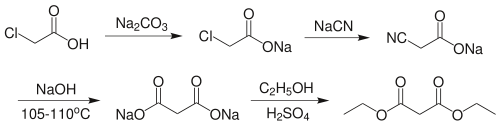
Preparation of Diethyl malonate: it can be prepared by reacting the sodium salt of chloroacetic acid with sodium cyanide, followed by base hydrolysis of the resultant nitrile to give the sodium salt malonic acid.
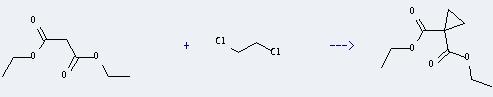
Uses of Diethyl malonate: One of the principle uses of this compound is in the malonic ester synthesis. And it can react with 1,2-dichloro-ethane to get cyclopropane-1,1-dicarboxylic acid diethyl ester. This reaction will need reagents K2CO3 and H2O, catalyst (n-Bu)4N(1+)Br(1-) and solvent benzene. The reaction time is 12 hours at reaction temperature of 80 °C. The yield is about 85%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. During using it, you should avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)CC(=O)OCC
(2)InChI: InChI=1/C7H12O4/c1-3-10-6(8)5-7(9)11-4-2/h3-5H2,1-2H3
(3)InChIKey: IYXGSMUGOJNHAZ-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 6400mg/kg (6400mg/kg) | Biochemical Journal. Vol. 34, Pg. 1196, 1940. | |
| rabbit | LD50 | skin | > 16mL/kg (16mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rat | LD50 | oral | 14900uL/kg (14.9mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. |
Related Products
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (hydroxymethyl)phosphonate
- Diethyl (methylthiomethyl)phosphonate
- Diethyl (phthalimidomethyl)phosphonate
- 10553-31-8
- 105533-75-3
- 105538-02-1
- 105538-07-6
- 105-54-4
- 105544-30-7
- 105544-36-3
- 10554-65-1
- 105-55-5
- 105558-26-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View