-
Name
Dipropylamine
- EINECS 205-565-9
- CAS No. 142-84-7
- Article Data114
- CAS DataBase
- Density 0.741 g/cm3
- Solubility soluble in water
- Melting Point -63 °C
- Formula C6H15N
- Boiling Point 108.8 °C at 760 mmHg
- Molecular Weight 101.192
- Flash Point 3.9 °C
- Transport Information UN 2383 3/PG 2
- Appearance clear, colorless liquid
- Safety 16-26-36/37/39-45
- Risk Codes 11-20/21/22-35
-
Molecular Structure
-
Hazard Symbols
 F,
F, C
C
- Synonyms Dipropylamine(8CI);Di-n-propylamine;Dipropanamine;N,N-Dipropylamine;N-Propyl-1-propanamine;n-Dipropylamine;
- PSA 12.03000
- LogP 1.78690
Synthetic route
-
-
25070-76-2
benzyl diallylcarbamate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In tetrahydrofuran-d8 at 20℃; for 24h; | 100% |
| With hydrogen In d(4)-methanol at 20℃; under 760.051 Torr; for 24h; | 100% |
| With hydrogen In methanol; d(4)-methanol at 40℃; under 760.051 Torr; for 1h; Reagent/catalyst; chemoselective reaction; | 100% |
| With hydrogen In d(4)-methanol at 20℃; under 2280.15 Torr; for 24h; Reagent/catalyst; chemoselective reaction; |
-
-
107-10-8
propylamine

-
-
74-85-1
ethene

-
-
201230-82-2
carbon monoxide

-
A
-
102-69-2
tri-n-propylamine

-
B
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| di(rhodium)tetracarbonyl dichloride In ethanol at 119℃; under 44253.5 Torr; for 1h; | A 1% B 99% |
| di(rhodium)tetracarbonyl dichloride In ethanol at 119℃; under 44253.5 Torr; for 1h; | A 2% B 98% |


-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; chloro-trimethyl-silane; magnesium In tetrahydrofuran at 50℃; for 24h; | 94% |
-
-
107-12-0
propiononitrile

-
A
-
102-69-2
tri-n-propylamine

-
B
-
7707-70-2
N-propylidenepropylamine

-
C
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With hydrogen at 220℃; | A 8.5% B 2.5% C 89% |
| With hydrogen at 240℃; Temperature; Flow reactor; | A 13% B 2.5% C 80% |

-
-
64935-06-4
3-Dipropylamino-7-hydroxy-heptanenitrile

-
A
-
75394-84-2
(+/-)-2-perhydro-2H-pyran-2-ylethanenitrile

-
B
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 180℃; | A 88% B 61% |

| Conditions | Yield |
|---|---|
| With isocyanate de chlorosulfonyle In diethyl ether for 10h; Ambient temperature; | 69% |
-
-
75162-84-4
1-dipropylamino-4-methyl-3-penten-1-ynamine

-
-
107-15-3
ethylenediamine

-
A
-
142-84-7
di-n-propylamine

-
B
-
87703-38-6
2-(3-methyl-2-butenyl)imidazoline

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane | A n/a B 68% |

-
-
75162-84-4
1-dipropylamino-4-methyl-3-penten-1-ynamine

-
-
141-43-5
ethanolamine

-
A
-
87703-36-4
2-(3-methyl-2-butenyl)oxazoline

-
B
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane at 100℃; for 4h; | A 65% B n/a |

-
-
70490-69-6
1-dipropylamino-3-penten-1-yne

-
-
107-15-3
ethylenediamine

-
A
-
142-84-7
di-n-propylamine

-
B
-
87703-37-5
2-(2-butenyl)imidazoline

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane | A n/a B 60% |


| Conditions | Yield |
|---|---|
| With ethanol; calcium at 20℃; for 24h; | 55% |
-
-
70490-69-6
1-dipropylamino-3-penten-1-yne

-
-
141-43-5
ethanolamine

-
A
-
87703-35-3
2-(2-butenyl)oxazoline

-
B
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane at 100℃; for 4h; | A 52% B n/a |

-
-
5848-64-6
Tris(di-n-propylamino)phosphine

-
-
131471-75-5
C14H11ClN4OS

-
A
-
15545-56-9
N-phenyl-N',N'-di(n-propyl)urea

-
B
-
131471-73-3
C25H45ClN6P2S

-
C
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| at 125℃; under 130 Torr; | A n/a B 11.5% C n/a |

-
-
5848-64-6
Tris(di-n-propylamino)phosphine

-
-
131471-74-4
1-(6-Methyl-2-benzothiazolyl)-4-phenylsemicarbazide

-
A
-
15545-56-9
N-phenyl-N',N'-di(n-propyl)urea

-
B
-
131471-70-0
C26H48N6P2S

-
C
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| at 125℃; under 130 Torr; | A n/a B 11% C n/a |


| Conditions | Yield |
|---|---|
| With ammonia at 420℃; ueber Silicagel; |

| Conditions | Yield |
|---|---|
| With alumina hydrate; ammonia at 370℃; under 139746 Torr; |

| Conditions | Yield |
|---|---|
| With diethyl ether at -10℃; zuletzt bei Siedetemperatur; |

| Conditions | Yield |
|---|---|
| With ethanol; ammonia; water at 100℃; under 5148.6 - 7355.08 Torr; | |
| With ethanol; ammonia; water at 100℃; under 5148.6 - 7355.08 Torr; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; hydrogen |

| Conditions | Yield |
|---|---|
| With chloroamine; Petroleum ether at -30℃; |

| Conditions | Yield |
|---|---|
| With ethanol; ammonia; water at 100℃; under 5148.6 - 7355.08 Torr; | |
| With ethanol; ammonia; water at 100℃; under 5148.6 - 7355.08 Torr; |

| Conditions | Yield |
|---|---|
| at 100℃; Rate constant; |

| Conditions | Yield |
|---|---|
| With nickel at 162 - 195℃; under 36775.4 - 73550.8 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With methanol; ammonia; nickel at 155 - 165℃; under 51485.6 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 40 - 50℃; under 4413.05 - 80905.8 Torr; Hydrogenation; | |
| With hydrogenchloride; iron at 100℃; | |
| With nickel-cobalt-cadmium-chromium at 390 - 400℃; | |
| With ethanol; nickel at 100℃; under 102971 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With Ni-doped silica; ammonia; hydrogen at 125℃; | |
| With Ni-doped silica; ammonia; hydrogen at 125℃; |

| Conditions | Yield |
|---|---|
| With ammonia; nickel at 100 - 150℃; under 73550.8 Torr; Hydrogenation; | |
| With hydrogenchloride; platinum under 2206.5 Torr; Hydrogenation; | |
| With 5%-palladium/activated carbon; ammonium formate In ethanol at 20℃; |

| Conditions | Yield |
|---|---|
| With hydrogen; nickel boride; silica gel In ethanol at 70℃; under 4560 Torr; Product distribution; Kinetics; other nickel catalyst, different times, solvents, and temp.; | |
| With hydrogen; nickel In methanol at 70℃; under 7500.6 Torr; Product distribution; also in presence of amines, competition with cyclohexene; | |
| With nickel at 130 - 140℃; Hydrogenation.unter Normaldruck; | |
| With ethanol; ammonia; nickel at 125℃; under 44130.5 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| at 100℃; Rate constant; |
-
-
60-29-7
diethyl ether

-
-
5775-34-8
N,N-Dipropyl-chloramine

-
-
6921-34-2
benzylmagnesium chloride

-
A
-
20441-12-7
N-benzyl-N,N-dipropylamine

-
B
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| at 5℃; |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; sodium nitrite In dichloromethane at 20℃; | 100% |
| With dinitrogen trioxide Kinetics; Rate constant; Thermodynamic data; ΔH(excit.); | |
| With hydrogenchloride; sodium nitrite |

| Conditions | Yield |
|---|---|
| di(rhodium)tetracarbonyl dichloride In ethanol at 115℃; under 37503 Torr; for 1.5h; | 100% |

-
-
142-84-7
di-n-propylamine

-
-
63587-45-1
Toluene-4-sulfonic acid 1-(toluene-4-sulfonyl)-piperidin-2-ylmethyl ester

-
-
186790-30-7
Dipropyl-[1-(toluene-4-sulfonyl)-piperidin-2-ylmethyl]-amine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene for 48h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In water Heating; | 100% |
-
-
142-84-7
di-n-propylamine

-
-
347888-67-9
N,N-di-n-propyl-4-[phenyl(exo-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)amino]benzamide

| Conditions | Yield |
|---|---|
| With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate Acylation; | 100% |
-
-
142-84-7
di-n-propylamine

-
-
287721-05-5
4-[phenyl(endo-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)amino]benzoic acid

-
-
287720-98-3
4-[(8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl)-phenyl-amino]-N,N-dipropyl-benzamide

| Conditions | Yield |
|---|---|
| With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate Acylation; | 100% |
-
-
24801-88-5
3-(triethoxypropyl) isocyanate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 3h; | 100% |
-
-
920490-05-7
6-(2-hydroxyethyl)-2-(methylthio)-4-pyrimidinyl 4-methyl-1-benzenesulfonate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 70℃; for 48h; | 100% |
-
-
1955-46-0
5-nitro-1,3-benzenedicarboxylic acid, monomethyl ester

-
-
142-84-7
di-n-propylamine

-
-
328284-59-9
5-nitro-N,N-dipropyl-isophthalamic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5-nitro-1,3-benzenedicarboxylic acid, monomethyl ester With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1h; Stage #2: di-n-propylamine In dichloromethane at 20℃; for 0.5h; | 100% |
| With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 5h; | 75% |
-
-
1071600-29-7
2-hydroxy-2-(4-methoxybenzoyl)cyclohexane-1-carboxylic acid

-
-
142-84-7
di-n-propylamine

-
-
1071600-59-3
2-hydroxy-2-(4-methoxy-benzoyl)-cyclohexanecarboxylate dipropyl-ammonium

| Conditions | Yield |
|---|---|
| In toluene at 65℃; Heating / reflux; | 100% |
-
-
1114592-18-5
tert-butyl (4-{[(6-chloropyrimidin-4-yl)carbonyl]amino}benzyl)carbamate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 100℃; for 2.5h; microwave irradiation; sealed tube; | 100% |
-
-
1338931-99-9
5,7-dichloro-6-methoxypyrazolo[1,5-a]pyrimidine

-
-
142-84-7
di-n-propylamine

-
-
1338932-08-3
(5-chloro-6-methoxypyrazolo[1,5-a]pyrimidin-7-yl)dipropylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; chemoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With water at 100℃; for 0.0833333h; Inert atmosphere; | 100% |

-
-
142-84-7
di-n-propylamine

-
-
1616610-11-7
N,N-dipropylnaphtho[2,3-d][1,3]dioxole-6-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: naphtho[2,3-d][1,3]dioxole-6-carboxylic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; Stage #2: di-n-propylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; | 100% |

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl 2-hydroxy-4-(2-oxoethyl)-2,3-dihydro-1H-indole-1-carboxylate; di-n-propylamine With acetic acid In dichloromethane for 0.75h; Stage #2: With sodium cyanoborohydride In dichloromethane for 2h; | 100% |
-
-
109687-65-2
(S)-(-)-1,1-dimethylethyl <3-methyl-1-<<(methylsulfonyl)oxy>methyl>butyl>carbamate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 40h; Inert atmosphere; | 100% |
-
-
126301-19-7, 134807-64-0, 109687-66-3
(2S)-2-[(tert-butoxycarbonyl)amino]-3-phenylpropyl methanesulfonate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 40h; Inert atmosphere; | 100% |
-
-
126301-16-4
(2S)-2-[(tert-butoxycarbonyl)amino]propyl methanesulfonate

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 40h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In ethanol for 2h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In benzene for 1h; Ambient temperature; | 99.5% |
-
-
142-84-7
di-n-propylamine

-
-
388071-08-7
methyl 3-bromo-5-(dipropylcarbamoyl)benzoate

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-5-(methoxycarbonyl)benzoic acid With HATU In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: di-n-propylamine In N,N-dimethyl-formamide at 20℃; | 99.5% |
| Multi-step reaction with 3 steps 1: 75 percent / CDI / CH2Cl2 / 5 h / 20 °C 2: 70 percent / H2 / Pd/C / 18 h / 2585.81 Torr 3: 75 percent / butyl nitrite; copper(II) bromide / acetonitrile / 2 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With oxygen In isopropyl alcohol at 50℃; under 1275.13 Torr; for 1.41667h; Autoclave; | 99.2% |
| Stage #1: carbon disulfide; di-n-propylamine With potassium hydroxide In tetrahydrofuran; water at 20℃; for 12h; Stage #2: With iodine In tetrahydrofuran; methanol; water at 20℃; for 1h; | 73% |
| With oxygen; eosin Y disodium salt In methanol under 3750.38 Torr; for 0.333333h; Solvent; Flow reactor; Irradiation; Green chemistry; | 69% |
-
-
10130-89-9
p-carboxyphenylsulfonyl chloride

-
-
142-84-7
di-n-propylamine

-
-
57-66-9
4-[(dipropylamino)sulfonyl]benzoic acid

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 18h; | 99% |
| With sodium hydrogencarbonate In water; acetone at 25℃; Schotten-Baumann Reaction; Flow reactor; | 78% |

| Conditions | Yield |
|---|---|
| In diethyl ether cooling; | 99% |
-
-
50-00-0
formaldehyd

-
-
142-84-7
di-n-propylamine

-
-
536-74-3
phenylacetylene

-
-
153396-18-0
3-phenyl-N,N-dipropylprop-2-yn-1-amine

| Conditions | Yield |
|---|---|
| With Cu(2-hydroxypyrimidinolate)2 In 1,4-dioxane at 39.84℃; for 21h; Inert atmosphere; | 99% |
| With copper(l) iodide In water; dimethyl sulfoxide at 30℃; for 5h; | 87% |
| With copper(l) iodide In 1,4-dioxane Heating; |

-
-
142-84-7
di-n-propylamine

-
-
86657-79-6
(4aS,9bR)-4-[1-Dimethylamino-meth-(E)-ylidene]-8,9b-dimethyl-1,4,4a,9b-tetrahydro-2H-dibenzofuran-3-one

-
-
86657-94-5
(4aS,9bR)-4-[1-Dipropylamino-meth-(E)-ylidene]-8,9b-dimethyl-1,4,4a,9b-tetrahydro-2H-dibenzofuran-3-one

| Conditions | Yield |
|---|---|
| In benzene for 8h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| at 100℃; for 0.0833333h; microwave irradiation; | 99% |
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.333333h; Ullmann condensation; ultrasonic irradiation; | 79% |
-
-
1878-69-9
3-iodophenylacetic acid

-
-
142-84-7
di-n-propylamine

-
-
865178-30-9
2-(3-iodo-phenyl)-N,N-dipropyl-acetamide

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 20h; | 99% |
Dipropylamine Chemical Properties
IUPAC: N-propylpropan-1-amine
Molecular Formula of Dipropylamine (CAS NO.142-84-7): C6H15N
Molecular Weight: 101.19
Synonyms of Dipropylamine (CAS NO.142-84-7): n,n-dipropylamine 1-Propanamine, N-propyl- ; Di-n-propylamine ; Dipropylamine ; Dipropylamine [UN2383] [Flammable liquid] ; RCRA waste no. U110 ; UN2383
Refractive index: n20/D 1.4049(lit.)
CAS NO: 142-84-7
Polar Surface Area: 3.24 Å2
Index of Refraction: 1.405
Molar Refractivity: 33.44 cm3
Molar Volume: 136.3 cm3
Surface Tension: 23 dyne/cm
Flash Point: 3.9 °C
Enthalpy of Vaporization: 33.47 kJ/mol
Index of Refraction: 1.405
Density: 0.741 g/cm3
Boiling Point: 108.8 °C at 760 mmHg
Vapour Pressure: 25.5 mmHg at 25°C
Stability: Stable. Highly flammable. Incompatible with strongoxidizing agents
The Structure of Dipropylamine (CAS NO.142-84-7):
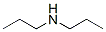
Dipropylamine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LC50 | inhalation | 4400mg/m3 (4400mg/m3) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 14, Pg. 80, 1975. | |
| mammal (species unspecified) | LD50 | unreported | 600mg/kg (600mg/kg) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 14, Pg. 80, 1975. | |
| mouse | LC50 | inhalation | 3070mg/m3/2H (3070mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 63, 1982. | |
| mouse | LD50 | unreported | 320mg/kg (320mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 35(4), Pg. 103, 1970. | |
| rabbit | LD50 | skin | 925mg/kg (925mg/kg) | National Technical Information Service. Vol. OTS0535430, | |
| rabbit | LD50 | subcutaneous | 1250mg/kg (1250mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 434, 1986. | |
| rat | LC50 | inhalation | 4400mg/m3/4H (4400mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 63, 1982. | |
| rat | LD50 | oral | 300mg/kg (300mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: MUSCLE WEAKNESS | National Technical Information Service. Vol. OTS0544865, |
| rat | LD50 | unreported | 280mg/kg (280mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 35(4), Pg. 103, 1970. | |
| rat | LDLo | intraperitoneal | 75mg/kg (75mg/kg) | Farmakologiya i Toksikologiya Vol. 31, Pg. 238, 1968. |
Dipropylamine Consensus Reports
Reported in EPA TSCA Inventory.
Dipropylamine Safety Profile
Poison by ingestion. Moderately toxic by skin contact and inhalation. A skin irritant. A very dangerous fire hazard, when exposed to heat or flame. Can react with oxidizers. Explosion hazard is unknown. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.
Safety Information of Dipropylamine (CAS NO.142-84-7):
Hazard Codes: F :  ,C
,C 
Risk Statements: 11-20/21/22-35 ( Highly Flammable ; Harmful by inhalation, in contact with skin and if swallowed ; Causes severe burns
Safety Statements 16-26-36/37/39-45 [Keep away from sources of ignition - No smoking ; In case of contact with eyes, rinse immediately with plenty of water and seek medical advice ; Wear suitable protective clothing, gloves and eye/face protection ; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) ] RIDADR UN 2383 3/PG 2
WGK Germany :1
RTECS : JL9200000
HazardClass :3
PackingGroup: II
Dipropylamine Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
Related Products
- Dipropylamine
- 142847-17-4
- 14284-87-8
- 14284-89-0
- 14284-92-5
- 14284-93-6
- 14284-94-7
- 142849-53-4
- 14284-95-8
- 14284-98-1
- 142851-03-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View