-
Name
Equol
- EINECS 208-522-2
- CAS No. 531-95-3
- Article Data38
- CAS DataBase
- Density 1.286 g/cm3
- Solubility Soluble in DMSO, methanol, or ethanol.
- Melting Point 189-190 °C
- Formula C15H14O3
- Boiling Point 441.7 °C at 760 mmHg
- Molecular Weight 242.274
- Flash Point 220.9 °C
- Transport Information
- Appearance white to off-white solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2H-1-Benzopyran-7-ol,3,4-dihydro-3-(4-hydroxyphenyl)-, (S)-;4',7-Isoflavandiol (6CI,7CI,8CI);(-)-(S)-Equol;(-)-Equol;(S)-(-)-4',7-Isoflavandiol;(S)-Equol;4',7-Dihydroxyisoflavan;Equol, (-)-;
- PSA 49.69000
- LogP 2.81650
Synthetic route
-
-
17238-05-0, 37054-07-2, 58865-02-4
dihydrodaidzein

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With Eggerthella sp. YY7918 at 37℃; for 72h; | 100% |

| Conditions | Yield |
|---|---|
| With Eggerthella sp. YY7918 at 37℃; for 72h; | 100% |
| With 5%-palladium/activated carbon; hydrogen; acetic acid In ethanol; water at 20℃; under 760.051 Torr; for 10h; | 95% |

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen In tetrahydrofuran; methanol; water at 20℃; for 0.75h; | 100% |
-
-
1208255-82-6
C36H29Br3O4

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Stage #1: C36H29Br3O4 With lithium aluminium tetrahydride In diethyl ether for 3h; Stage #2: With water In diethyl ether Cooling with ice; | 95% |
-
-
922179-56-4
(S)-7-(methoxymethoxy)-3-(4'-methoxymethoxy)-phenylchroman

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol In dichloromethane at 4.8 - 20℃; for 6h; | 93% |
| With hydrogenchloride In tetrahydrofuran; methanol; water at 60℃; for 0.333333h; Inert atmosphere; | 92% |
-
-
1383121-33-2
(S)-4-(3-bromo-2-(4-hydroxyphenyl)propyl)benzene-1,3-diol

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 50℃; for 8h; | 91% |
| With potassium carbonate In acetone at 50℃; for 8h; | 110 mg |

| Conditions | Yield |
|---|---|
| With pyridine at 150℃; Inert atmosphere; | 88% |
| With pyridine hydrogenfluoride In neat (no solvent) at 150℃; for 48h; Inert atmosphere; | 82% |
| With pyridine hydrochloride at 160℃; for 48h; Inert atmosphere; Schlenk technique; | 72% |
| With pyridine hydrochloride at 150℃; for 28h; | 66% |
-
-
1404301-87-6
(S)-7-methoxy-3-(4-methoxyphenyl)spiro[chroman-2,2'-[1,3]dithiane]

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-7-methoxy-3-(4-methoxyphenyl)spiro[chroman-2,2'-[1,3]dithiane] With hydrogen Stage #2: With pyridine; hydrogenchloride at 150℃; | 63% |

| Conditions | Yield |
|---|---|
| at 180℃; |

| Conditions | Yield |
|---|---|
| With ammonium formate; acetic acid; palladium dihydroxide for 1h; Heating; | |
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; ammonium formate / methanol / 4 h / 50 °C / Inert atmosphere 2: [(1S,2S)-N-(p-toluensulfonyl)-1,2-diphenylethanediamine](p-cymene)ruthenium (I); triethylamine; hydrogen / methanol / 19 h / 60 °C / 7500.75 Torr / Inert atmosphere; Autoclave 3: palladium 10% on activated carbon; acetic acid; hydrogen / methanol / 4 h / 60 °C / 5250.53 Torr / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; ethanethiol In dichloromethane at 0℃; for 1h; |

| Conditions | Yield |
|---|---|
| In ethanol; hexane for 0 - 0.283333h; Resolution of racemate; | |
| With CYCLOBOND I 2000 RSP In formic acid; water; acetonitrile Resolution of racemate; | |
| With cellulose (3,5-dimethylphenylcarbamate) coated reduced graphene oxide(at)silica gel In hexane; isopropyl alcohol at 25℃; Resolution of racemate; enantioselective reaction; |
-
-
917379-11-4
(S)-3-(2-bromo-4-methoxyphenyl)-2-(4-methoxyphenyl)propan-1-ol

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 46 percent / 2-(di-tert-butylphosphino)-1,1'-binaphthyl; Cs2CO3; Pd(OAc)2 / toluene / 22 h / 50 °C 2: 66 percent / pyridine hydrochloride / 28 h / 150 °C View Scheme |
-
-
917379-10-3
(S)-4-benzyl-3-((S)-3-(2-bromo-4-methoxyphenyl)-2-(4-methoxyphenyl)propanoyl)-2-oxazolidinone

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / LiAlH4 / tetrahydrofuran / 0.25 h / cooling 2: 46 percent / 2-(di-tert-butylphosphino)-1,1'-binaphthyl; Cs2CO3; Pd(OAc)2 / toluene / 22 h / 50 °C 3: 66 percent / pyridine hydrochloride / 28 h / 150 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: SOCl2 / 2 h / Heating 2: 237 g / n-BuLi / tetrahydrofuran; hexane / -65 - 20 °C 3: 167.4 g / NaHMDS / tetrahydrofuran / 0.17 h / cooling 4: 96 percent / LiAlH4 / tetrahydrofuran / 0.25 h / cooling 5: 46 percent / 2-(di-tert-butylphosphino)-1,1'-binaphthyl; Cs2CO3; Pd(OAc)2 / toluene / 22 h / 50 °C 6: 66 percent / pyridine hydrochloride / 28 h / 150 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: acetic anhydride; triethylamine / 2 h / Reflux 2.1: C56H72IrNOP; hydrogen; triethylamine / methanol / 72 h / 70 °C / 9120.61 Torr 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 2.25 h / 0 - 20 °C 3.2: 0 °C 4.1: carbon tetrabromide; triphenylphosphine / dichloromethane / 3 h / 20 °C / Cooling with ice 5.1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 6.1: potassium carbonate / acetone / 8 h / 50 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: oxalyl dichloride / benzene / 24 h / 20 °C 2.1: potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate / dichloromethane; water / 20 °C 3.1: hydrogen; 10% Pd/C / ethyl acetate / 20 °C / 750.08 Torr 4.1: trimethylaluminum / dichloromethane; hexane / 0 - 20 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 6.1: hydrogen 6.2: 150 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: thionyl chloride / 2 h / Inert atmosphere 2.1: n-butyllithium / tetrahydrofuran / 2 h / -65 - -45 °C / Inert atmosphere 3.1: N-ethyl-N,N-diisopropylamine; di-n-butylboryl trifluoromethanesulfonate / dichloromethane / 3.5 h / -25 - -15 °C / Inert atmosphere 3.2: 1.83 h / -25 - 15 °C / Inert atmosphere 4.1: triethylsilane; trifluoroacetic acid / dichloromethane / 0.67 h / 0 - 20 °C / Inert atmosphere 5.1: hydrogenchloride / methanol / 0.5 h / Reflux; Inert atmosphere 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 0 - 20 °C / Inert atmosphere 7.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 6 h / 20 °C / Inert atmosphere 8.1: pyridine / 150 °C / Inert atmosphere View Scheme |
-
-
143589-97-3
(4S)-3-<2-(4-Methoxyphenyl)-1-oxoethyl>-4-(phenylmethyl)-2-oxazolidinone

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 167.4 g / NaHMDS / tetrahydrofuran / 0.17 h / cooling 2: 96 percent / LiAlH4 / tetrahydrofuran / 0.25 h / cooling 3: 46 percent / 2-(di-tert-butylphosphino)-1,1'-binaphthyl; Cs2CO3; Pd(OAc)2 / toluene / 22 h / 50 °C 4: 66 percent / pyridine hydrochloride / 28 h / 150 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 237 g / n-BuLi / tetrahydrofuran; hexane / -65 - 20 °C 2: 167.4 g / NaHMDS / tetrahydrofuran / 0.17 h / cooling 3: 96 percent / LiAlH4 / tetrahydrofuran / 0.25 h / cooling 4: 46 percent / 2-(di-tert-butylphosphino)-1,1'-binaphthyl; Cs2CO3; Pd(OAc)2 / toluene / 22 h / 50 °C 5: 66 percent / pyridine hydrochloride / 28 h / 150 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate / dichloromethane; water / 20 °C 2.1: hydrogen; 10% Pd/C / ethyl acetate / 20 °C / 750.08 Torr 3.1: trimethylaluminum / dichloromethane; hexane / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 5.1: hydrogen 5.2: 150 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: n-butyllithium / tetrahydrofuran / 2 h / -65 - -45 °C / Inert atmosphere 2.1: N-ethyl-N,N-diisopropylamine; di-n-butylboryl trifluoromethanesulfonate / dichloromethane / 3.5 h / -25 - -15 °C / Inert atmosphere 2.2: 1.83 h / -25 - 15 °C / Inert atmosphere 3.1: triethylsilane; trifluoroacetic acid / dichloromethane / 0.67 h / 0 - 20 °C / Inert atmosphere 4.1: hydrogenchloride / methanol / 0.5 h / Reflux; Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 0 - 20 °C / Inert atmosphere 6.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 6 h / 20 °C / Inert atmosphere 7.1: pyridine / 150 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 68 percent / acetic acid; ammonium formate / Pd(OH)2 / 1 h / Heating 2: aluminum trichloride; ethane thiol / CH2Cl2 / 1 h / 0 °C View Scheme |

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone |
-
-
486-66-8
daidzein

-
A
-
17238-05-0, 37054-07-2, 58865-02-4
dihydrodaidzein

-
C
-
81267-65-4
idronoxil

-
D
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen; acetic acid In ethanol at 20℃; for 10h; |

-
-
1057663-19-0
(S)-3-(2,4-dimethoxyphenyl)-2-(4-methoxyphenyl)propan-1-ol

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: carbon tetrabromide; triphenylphosphine / dichloromethane / 3 h / 20 °C / Cooling with ice 2: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 3: potassium carbonate / acetone / 8 h / 50 °C View Scheme |
-
-
1057663-20-3
(S)-1-(3-bromo-2-(4-methoxyphenyl)propyl)-2,4-dimethoxy-benzene

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 2: potassium carbonate / acetone / 8 h / 50 °C View Scheme | |
| Stage #1: (S)-1-(3-bromo-2-(4-methoxyphenyl)propyl)-2,4-dimethoxy-benzene With boron tribromide In dichloromethane at -78 - 20℃; for 19h; Inert atmosphere; Stage #2: With potassium carbonate In acetone at 50℃; for 8h; | 63.1 mg |
-
-
1383121-29-6
(S)-3-(2,4-dimethoxyphenyl)-2-(4-methoxyphenyl)propionic acid

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 2.25 h / 0 - 20 °C 1.2: 0 °C 2.1: carbon tetrabromide; triphenylphosphine / dichloromethane / 3 h / 20 °C / Cooling with ice 3.1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 4.1: potassium carbonate / acetone / 8 h / 50 °C View Scheme |
-
-
73867-36-4
(E)-3-(2,4-dimethoxyphenyl)-2-(4-methoxyphenyl)acrylic acid

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: C56H72IrNOP; hydrogen; triethylamine / methanol / 72 h / 70 °C / 9120.61 Torr 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 2.25 h / 0 - 20 °C 2.2: 0 °C 3.1: carbon tetrabromide; triphenylphosphine / dichloromethane / 3 h / 20 °C / Cooling with ice 4.1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 5.1: potassium carbonate / acetone / 8 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: acetic anhydride; triethylamine / 2 h / Reflux 2.1: C56H72IrNOP; hydrogen; triethylamine / methanol / 72 h / 70 °C / 9120.61 Torr 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 2.25 h / 0 - 20 °C 3.2: 0 °C 4.1: carbon tetrabromide; triphenylphosphine / dichloromethane / 3 h / 20 °C / Cooling with ice 5.1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C 6.1: potassium carbonate / acetone / 8 h / 50 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: pyridine; piperidine / 3.5 h / 125 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / Reflux; Inert atmosphere 3.1: pyridinium chlorochromate / dichloromethane / 1 h / 20 °C / Molecular sieve 4.1: copper(I) bromide; sodium hydrogencarbonate; (2R,5R)-2-tert-butyl-3-methyl-5-phenyl-4-imidazolidinone trichloroacetic acid salt / toluene; diethyl ether / 44 h / 20 °C 5.1: sodium tetrahydroborate / methanol; dichloromethane; toluene; diethyl ether / 1 h / 23 °C 5.2: 3 h / 0 - 20 °C 6.1: boron tribromide / dichloromethane / 19 h / -78 - 20 °C / Inert atmosphere 6.2: 8 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogen; 10% Pd/C / ethyl acetate / 20 °C / 750.08 Torr 2.1: trimethylaluminum / dichloromethane; hexane / 0 - 20 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 4.1: hydrogen 4.2: 150 °C View Scheme |
-
-
1404301-54-7
7-methoxy-3-(4-methoxyphenyl)hydrocoumarin

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: trimethylaluminum / dichloromethane; hexane / 0 - 20 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 3.1: hydrogen 3.2: 150 °C View Scheme |
-
-
1404301-69-4
2-(2-(1,3-dithian-2-ylidene)-2-(4-methoxyphenyl)ethyl)-5-methoxyphenol

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 2.1: hydrogen 2.2: 150 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate / dichloromethane; water / 20 °C 2.1: hydrogen; 10% Pd/C / ethyl acetate / 20 °C / 750.08 Torr 3.1: trimethylaluminum / dichloromethane; hexane / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: (R)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-1,1'-binaphthyl phosphate / cyclohexane / 60 h / 20 °C / Molecular sieve 5.1: hydrogen 5.2: 150 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 0.5 h / Cooling with ice; Inert atmosphere 1.2: 20 h / Inert atmosphere 2.1: N-ethyl-N,N-diisopropylamine; di-n-butylboryl trifluoromethanesulfonate / dichloromethane / 3.5 h / -25 - -15 °C / Inert atmosphere 2.2: 1.83 h / -25 - 15 °C / Inert atmosphere 3.1: triethylsilane; trifluoroacetic acid / dichloromethane / 0.67 h / 0 - 20 °C / Inert atmosphere 4.1: hydrogenchloride / methanol / 0.5 h / Reflux; Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 0 - 20 °C / Inert atmosphere 6.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 6 h / 20 °C / Inert atmosphere 7.1: pyridine / 150 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; Schlenk technique; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With recombinant O-prenyltransferase from Antrodia camphorata In aq. buffer at 37℃; for 12h; pH=7; Enzymatic reaction; | A 27.3% B 15.6% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
531-95-3
equol
-
-
531-95-3
equol

-
-
21140-91-0
(-)-Di-O-acetyl-equol

| Conditions | Yield |
|---|---|
| at 240℃; |

-
-
75-77-4
chloro-trimethyl-silane

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
531-95-3
equol

-
-
81910-32-9
C21H30O3Si2

| Conditions | Yield |
|---|---|
| With pyridine at 65℃; for 0.5h; |

-
-
77377-52-7
N-methyl-N-tert-butyldimethylsilyl-1,1,1-trifluoroacetamide

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
531-95-3
equol

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 4h; |
EQUOL Chemical Properties
IUPAC Name: (3S)-3-(4-Hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol
The MF of Equol (CAS NO.531-95-3) is C15H14O3.
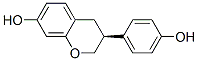
The MW of Equol (CAS NO.531-95-3) is 242.27.
Synonyms of Equol (CAS NO.531-95-3): (3S)-3-(4-Hydroxyphenyl)-7-chromanol ; 2H-1-Benzopyran-7-ol, 3,4-dihydro-3-(4-hydroxyphenyl)-, (3S)- ; 3,4-Dihydro-3-(4-hydroxyphenyl)-(S)-2H-1-benzopyran-7-ol ; 7-Hydroxy-3-(4'-hydroxyphenyl)chroman
Product Categories: Iso-Flavones;Chiral Reagents;Heterocycles;Metabolites & Impurities
Apperance: White to Off-White Solid
Index of Refraction: 1.644
EINECS: 208-522-2
Density: 1.286 g/ml
Flash Point: 220.9 °C
Boiling Point: 441.7 °C
Melting Point: 189-190 °C
EQUOL Uses
Equol (CAS NO.531-95-3) is used as receptor and binding activity.A human urinary metabolite of Daidzein. It is also a natural estrogenic metabolite from soy isoflavones.
EQUOL Toxicity Data With Reference
| 1. | msc-hmn-oth 1 µmol/L/24H | CALEDQ Cancer Letters (Shannon, Ireland). 142 (1999),111. |
EQUOL Safety Profile
Mutation data reported. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating vapors.Safety information of Equol (CAS NO.531-95-3):
WGK Germany 3
F 3-10
EQUOL Specification
Equol (4',7-isoflavandiol) is an isoflavandiol metabolized from daidzein, a type of isoflavone, by bacterial flora in the intestinesWhile endogenous estrogenic hormones such as estradiol are steroids, equol is a nonsteroidal estrogen. However, only about 30-50% of people have intestinal bacteria that make equol.Equol may have beneficial effects on the incidence of prostate cancer and physiological changes after menopause. Other benefits may be realized in treating male pattern baldness, acne, and other problems because it functions as a DHT blocker. S-Equol preferentially activates estrogen receptor type β.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View