-
Name
Ethyl 6-chloronicotinate
- EINECS -0
- CAS No. 49608-01-7
- Article Data12
- CAS DataBase
- Density 1.246 g/cm3
- Solubility
- Melting Point 26-30 °C(lit.)
- Formula C8H8ClNO2
- Boiling Point 249.207 °C at 760 mmHg
- Molecular Weight 185.61
- Flash Point 104.517 °C
- Transport Information
- Appearance white crystal powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Chloropyridine-5-carboxylicacid ethyl ester;6-Chloronicotinic acid ethyl ester;Ethyl 2-chloro-5-pyridinecarboxylate;Ethyl 6-chloro-3-nicotinate;Ethyl 6-chloro-3-pyridinecarboxylate;
- PSA 39.19000
- LogP 1.91170
Ethyl 6-chloronicotinate Specification
The Ethyl 6-chloronicotinate, with the CAS registry number 49608-01-7, is also known as 6-Chloronicotinic acid ethyl ester. It belongs to the product categories of Esters; Pyridines; Building Blocks; C7 to C18; C8 to C9; Chemical Synthesis; Halogenated Heterocycles; Heterocyclic Building Blocks. This chemical's molecular formula is C8H8ClNO2 and molecular weight is 185.61. What's more, its IUPAC name is called ethyl 6-Ehloropyridine-3-carboxylate.
Physical properties about Ethyl 6-chloronicotinate are: (1)ACD/LogP: 1.826; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.83; (4)ACD/LogD (pH 7.4): 1.83; (5)ACD/BCF (pH 5.5): 14.38; (6)ACD/BCF (pH 7.4): 14.38; (7)ACD/KOC (pH 5.5): 234.65; (8)ACD/KOC (pH 7.4): 234.65; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 39.19 Å2; (13)Index of Refraction: 1.525; (14)Molar Refractivity: 45.645 cm3; (15)Molar Volume: 149.007 cm3; (16)Polarizability: 18.095×10-24cm3; (17)Surface Tension: 43.160 dyne/cm; (18)Density: 1.246 g/cm3; (19)Flash Point: 104.517 °C; (20)Enthalpy of Vaporization: 48.643 kJ/mol; (21)Boiling Point: 249.207 °C at 760 mmHg; (22)Vapour Pressure: 0.023 mmHg at 25 °C.
Preparation of Ethyl 6-chloronicotinate: this chemical can be prepared by 6-chloro-nicotinic acid with ethanol. The reaction occurs with reagent H2SO4 and other condition of heating for 2 hours. The yield is 46 %.

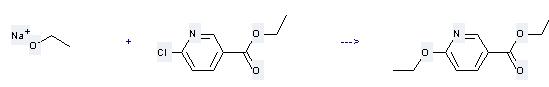
Uses of Ethyl 6-chloronicotinate: it is used to produce other chemicals. For example, it can react with sodium ethoxide to get 6-ethoxy-nicotinic acid ethyl ester. The reaction occurs with reagent EtOH and other condition of heating for 16 hours. The yield is 77 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: CCOC(=O)c1ccc(nc1)Cl
(2) InChI: InChI=1S/C8H8ClNO2/c1-2-12-8(11)6-3-4-7(9)10-5-6/h3-5H,2H2,1H3
(3) InChIKey: ILDJJTQWIZLGPO-UHFFFAOYSA-N
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- Ethyl (4-oxo-3,4-dihydrophthalazin-1-yl)acetate
- Ethyl (5-fluoro-2-iodophenoxy)acetate
- Ethyl (6-amino-9H-purin-9-yl)acetate
- Ethyl (6-aminopyridin-2-yl)acetate
- Ethyl (benzyloxy)acetate
- Ethyl (chlorosulfonyl)acetate
- Ethyl (E)-crotonate
- 4960-82-1
- 49609-84-9
- 496-10-6
- 496-11-7
- 496-12-8
- 496-13-9
- 496-14-0
- 496-15-1
- 496-16-2
- 49617-83-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View