-
Name
ACETIC ACID
- EINECS 200-580-7
- CAS No. 55896-93-0
- Article Data20
- CAS DataBase
- Density 1.43 g/cm3
- Solubility
- Melting Point 16.2 °C(lit.)
- Formula C4H7ClO4S
- Boiling Point 244.28 °C at 760 mmHg
- Molecular Weight 186.616
- Flash Point 101.537 °C
- Transport Information UN 2790 8/PG 2
- Appearance
- Safety 23-26-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Acetic acid,(chlorosulfonyl)-, ethyl ester (9CI);(Chlorosulfonyl)acetic acid ethyl ester;2-(Chlorosulfonyl)acetic acid ethyl ester;Ethoxycarbonylmethanesulfonylchloride;Ethyl 2-(chlorosulfonyl)acetate;Ethyl2-(chlorosulfonyl)ethanoate;
- PSA 68.82000
- LogP 1.19890
Synthetic route
-
-
22128-42-3
sodium 2-ethoxy-2-oxoethane-1-sulfonate

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 80℃; for 2h; | 92% |
| With phosphorus pentachloride at 100℃; for 0.75h; Inert atmosphere; | 85% |
| With phosphorus pentachloride at 20℃; for 0.333333h; | 65% |
-
-
89124-45-8
acetic acid ethylester sulfonic acid

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| With trichlorophosphate | 80% |
| With thionyl chloride at 120℃; | |
| With trichlorophosphate at 125℃; | |
| With trichlorophosphate | |
| With trichlorophosphate |
-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| With water; chlorine In dichloromethane at 30℃; for 1h; Cooling with acetone-dry ice; | 70% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: chloroacetic acid ethyl ester With sodium sulfite In ethanol; water for 6h; Heating; Stage #2: With oxalyl dichloride In N,N-dimethyl-formamide; toluene at 100℃; for 3h; Further stages.; | 59% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 3h; Inert atmosphere; | 55% |
| at -12℃; | |
| In benzene for 1h; Heating; |

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
470477-78-2
ethyl N-[4-(1-methylpiperidin-4-yloxy)-3-trifluoromethylphenyl]sulfamoylacetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 1h; | 100% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; Inert atmosphere; | 100% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
877-80-5
N-methyl-4-nitrobenzylideneamine

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 20℃; for 24h; Reagent/catalyst; Staudinger Ketene Cycloaddition; diastereoselective reaction; | 99% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
96649-05-7
3-(methoxymethoxy)aniline

-
-
1049708-54-4
{N-[3-(methoxymethoxy)phenyl]sulfamoyl}acetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 2.5h; | 97% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 96% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 96% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 96% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
2344-70-9, 14786-43-7, 22148-86-3, 39516-03-5
(R)-4-phenyl-2-butanol

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 95% |
-
-
337520-59-9
4-(1-t-butoxycarbonylpiperidin-4-yloxy)-3,5-dichloroaniline

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
337520-60-2
ethyl N-[4-(1-t-butoxycarbonylpiperidin-4-yloxy)-3,5-dichlorophenyl]sulfamoylacetate

| Conditions | Yield |
|---|---|
| With pyridine In hexane; dichloromethane; ethyl acetate | 95% |
| With pyridine In dichloromethane |
-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
1201917-28-3
ethyl (3S)-2-[[(3,7-dimethyl-6-octen-1-yl)oxy]sulfonyl]-acetate

| Conditions | Yield |
|---|---|
| With triethylamine | 95% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 95% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
1192-37-6
methylenecyclohexane

-
-
1354702-92-3
ethyl 3-(1-chlorocyclohexyl)propanoate

| Conditions | Yield |
|---|---|
| With di(undecanoyl) peroxide In benzene Inert atmosphere; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 93% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
540-37-4
p-aminoiodobenzene

-
-
658709-20-7
(4-iodo-phenylsulfamoyl)-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate; p-aminoiodobenzene With triethylamine In ethyl acetate at 20℃; for 72h; Stage #2: With hydrogenchloride In water; ethyl acetate | 92% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
121004-44-2
N-methyl-1-(3-nitrophenyl)methanimine

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 20℃; for 24h; Reagent/catalyst; Staudinger Ketene Cycloaddition; diastereoselective reaction; | 92% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
40230-24-8
2-amino-4,6-diphenylpyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 2-amino-4,6-diphenylpyrimidine In tetrahydrofuran at 25℃; for 0.25h; Solvent; Sonication; Green chemistry; | 92% |
| Stage #1: 2-amino-4,6-diphenylpyrimidine With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 69% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
1226780-87-5
2-amino-4-(4-bromophenyl)-6-(4-bromophenyl)pyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 2-amino-4-(4-bromophenyl)-6-(4-bromophenyl)pyrimidine In tetrahydrofuran at 25℃; for 0.283333h; Sonication; Green chemistry; | 92% |
| Stage #1: 2-amino-4-(4-bromophenyl)-6-(4-bromophenyl)pyrimidine With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 68% |
-
-
928-92-7
(E)-hex-4-en-1-ol

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; Inert atmosphere; | 92% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
13159-16-5
(E)-5-phenyl-4-penten-1-ol

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; Inert atmosphere; | 92% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
2104-09-8
4-(4-nitro-phenyl)-thiazol-2-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 4-(4-nitro-phenyl)-thiazol-2-ylamine In tetrahydrofuran at 25℃; for 0.216667h; Sonication; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 91% |

| Conditions | Yield |
|---|---|
| With di(undecanoyl) peroxide In benzene Inert atmosphere; Reflux; | 91% |
-
-
2010-06-2
2-Amino-4-phenylthiazole

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 2-Amino-4-phenylthiazole In tetrahydrofuran at 25℃; for 0.316667h; Sonication; Green chemistry; | 91% |
| Stage #1: 2-Amino-4-phenylthiazole With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 72% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: C16H11N5O4 In tetrahydrofuran at 25℃; for 0.316667h; Sonication; Green chemistry; | 91% |
| Stage #1: C16H11N5O4 With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 70% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
60472-20-0
4-(4-methoxyphenyl)-1H-imidazol-2-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 4-(4-methoxyphenyl)-1H-imidazol-2-ylamine In tetrahydrofuran at 25℃; for 0.25h; Sonication; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran | 90% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
141-78-6
ethyl acetate

-
-
337520-13-5
4-(1-t-butoxycarbonylpiperidin-4-yloxy)-3-ethoxycarbonylaniline

-
-
337520-14-6
ethyl N-[4-(1-t-butoxycarbonylpiperidin-4-yloxy)-3-ethoxycarbonylphenyl]sulfamoylacetate

| Conditions | Yield |
|---|---|
| With pyridine In hexane; dichloromethane | 90% |

-
-
2103-99-3
4-(4-chlorophenyl)-2-thiazolamine

-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 4-(4-chlorophenyl)-2-thiazolamine In tetrahydrofuran at 25℃; for 0.333333h; Sonication; Green chemistry; | 90% |
| Stage #1: 4-(4-chlorophenyl)-2-thiazolamine With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 75% |
-
-
55896-93-0
ethyl 2-(chlorosulfonyl)acetate

-
-
6775-40-2
2-amino-4-phenylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(chlorosulfonyl)acetate With sodium In tetrahydrofuran at 25℃; for 0.0833333h; Sonication; Green chemistry; Stage #2: 2-amino-4-phenylimidazole In tetrahydrofuran at 25℃; for 0.25h; Sonication; Green chemistry; | 90% |
| Stage #1: 2-amino-4-phenylimidazole With dmap; triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: ethyl 2-(chlorosulfonyl)acetate In dichloromethane at 40℃; Inert atmosphere; | 71% |
| With dmap; triethylamine In dichloromethane |
Ethyl (chlorosulfonyl)acetate Chemical Properties
Product Name: Ethyl (chlorosulfonyl)acetate (CAS NO.55896-93-0)
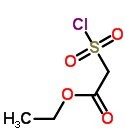
Molecular Formula: C4H7ClO4S
Molecular Weight: 186.614g/mol
Mol File: 55896-93-0.mol
Boiling point: 244.28 °C at 760 mmHg
Flash Point: 101.537 °C
Density: 1.43 g/cm3
Surface Tension: 44.87 dyne/cm
Enthalpy of Vaporization: 48.133 kJ/mol
Vapour Pressure: 0.031 mmHg at 25°C
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 55897-64-8
- 55897-66-0
- 55898-15-2
- 55898-43-6
- 5589-84-4
- 55898-77-6
- 55899-42-8
- 5589-96-8
- 5590-18-1
- 55902-93-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View