-
Name
Ethyl Hexanoate
- EINECS 204-640-3
- CAS No. 123-66-0
- Article Data115
- CAS DataBase
- Density 0.878 g/cm3
- Solubility INSOLUBLE
- Melting Point -67 °C
- Formula C8H16O2
- Boiling Point 167.9 °C at 760 mmHg
- Molecular Weight 144.214
- Flash Point 49.4 °C
- Transport Information UN 3272 3/PG 3
- Appearance clear colourless liquid
- Safety 16-26-36
- Risk Codes 10-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Caproicacid ethyl ester;Hexanoic acid, monoethyl ester (9CI);Ethyl hexanoate;Hxanoic acid, ethyl ester;Ethylhexanoat;
- PSA 26.30000
- LogP 2.12980
Synthetic route

| Conditions | Yield |
|---|---|
| With Oxone at 20℃; for 18h; | 95% |
| With oxygen at 100℃; under 3750.38 Torr; for 5h; Autoclave; |

| Conditions | Yield |
|---|---|
| zirconium(IV) oxide for 5h; Heating; | 100% |
| With 4-methyl-morpholine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride for 4h; | 93% |
| With 8-hydroxyquinoline methanesulfonate at 85℃; for 3h; Temperature; Reagent/catalyst; Concentration; | 91.4% |

| Conditions | Yield |
|---|---|
| Stage #1: penta-1,3-diene; ethanol With tris-(dibenzylideneacetone)dipalladium(0); methanesulfonic acid; 1,2-bis[di(t-butyl)phosphinomethyl]benzene In methanol for 0.25h; Sonication; Schlenk technique; Stage #2: carbon monoxide With hydrogen In methanol under 22502.3 Torr; |


| Conditions | Yield |
|---|---|
| With D-(+)-glucose In aq. buffer at 15℃; for 16h; Overall yield = 44.3 %; | |
| With glucose dehydrogenase; D-glucose; potassium chloride; NADPH In aq. buffer at 30℃; pH=8.5; Baeyer-Villiger Ketone Oxidation; Enzymatic reaction; regioselective reaction; |
-
-
175020-60-7
ethyl 2-(pyridin-2-ylsulfonyl)hexanoate

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 1h; desulfonylation; Heating; | 91% |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 36h; Heating; | 60% |
| Multi-step reaction with 2 steps 1: 61 percent / m-CPBA / CHCl3; CH2Cl2 2: 12 percent / Bu3SnH; AIBN / benzene / 1 h / Heating View Scheme |
-
-
64-17-5
ethanol

-
-
111-26-2
hexan-1-amine

-
-
201230-82-2
carbon monoxide

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
539-82-2
ethyl n-valerate

-
C
-
106-30-9
ethyl heptanoate

-
D
-
7451-47-0
N-hexylcarbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| With oxygen; Sulfate; zirconium(IV) oxide; palladium dichloride at 170℃; under 45003.6 Torr; for 3h; Further byproducts given. Title compound not separated from byproducts; | A n/a B n/a C n/a D 81% |


| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; cobalt(II) chloride; lithium iodide In tetrahydrofuran at 10℃; for 1h; | 78% |
-
-
2396-84-1
(2E,4E)-ethyl hexa-2,4-dienoate

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
27829-72-7
ethyl (E)-hex-2-enoate

-
C
-
26553-46-8
ethyl (E)-3-hexenoate

-
D
-
64187-83-3
ethyl (3Z)-hex-3-enoate

| Conditions | Yield |
|---|---|
| With hydrogen; silica-supported [(CH2)3-N(CH2PPh2)2][Pd(dba)] In methanol at 25℃; under 724.007 Torr; for 1h; Title compound not separated from byproducts; | A 7 % Chromat. B 86 % Chromat. C 4 % Chromat. D 3 % Chromat. |


| Conditions | Yield |
|---|---|
| With hydrogen at 80℃; under 7500.75 Torr; for 1h; Autoclave; | 92% |
-
-
27829-72-7
ethyl (E)-hex-2-enoate

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 150℃; under 18751.9 Torr; for 15h; Sealed tube; | |
| Stage #1: ethyl (E)-hex-2-enoate With (+)-1,2-bis((2S,5S)-2,5-diphenylphospholanyl)ethane; copper diacetate at 20℃; for 24h; Inert atmosphere; Stage #2: With sodium hydrogencarbonate In water; ethyl acetate at 20℃; for 0.416667h; Inert atmosphere; Cooling with ice; |
-
-
175020-61-8
ethyl 2-(pyrimidin-2-ylsulfonyl)hexanoate

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 1h; desulfonylation; Heating; | 100% |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 1h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; tert.-butylnitrite; iron(II) phthalocyanine; hydrogen In ethanol at 20℃; under 7500.75 Torr; for 3h; |

| Conditions | Yield |
|---|---|
| With zinc chromite; hydrogen at 360℃; under 154457 Torr; und folgenden Hydrieren an Nickel; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether In ethanol; benzene 1.) room temperature, 1.5 h, 2.) reflux, 23 h; | 84% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In diethyl ether at 25℃; | 99 % Chromat. |
| With Pd(OAc)2 dopped triglycidyl 1-ethyl-3-methylimidazoliumacetate polyether In methanol at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / NaH / dimethylformamide / 0 - 20 °C 2: 5.15 g / m-CPBA / CHCl3; CH2Cl2 / 20 h / -20 - 20 °C 3: 100 percent / Bu3SnH; AIBN / benzene / 1 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: NaH / tetrahydrofuran; dimethylformamide 2: m-CPBA 3: 60 percent / Bu3SnH, AIBN / benzene / 36 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: NaH / tetrahydrofuran; dimethylformamide 2: m-CPBA 3: Bu3SnH, AIBN / benzene / 1 h / Heating View Scheme |

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 5.07 g / m-CPBA / CHCl3; CH2Cl2 / -20 - 20 °C 2: 91 percent / Bu3SnH; AIBN / toluene / 1 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 5.07 g / m-CPBA / CHCl3; CH2Cl2 / -20 - 20 °C 2: 61 percent / m-CPBA / CHCl3; CH2Cl2 3: 12 percent / Bu3SnH; AIBN / benzene / 1 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: m-CPBA 2: 60 percent / Bu3SnH, AIBN / benzene / 36 h / Heating View Scheme |
-
-
26553-46-8
ethyl (E)-3-hexenoate

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| With ethanol; lithium; nickel dichloride; 4,4'-di-tert-butylbiphenyl In tetrahydrofuran at 20℃; for 24h; | 86 % Chromat. |
-
-
288400-57-7
2-(Pyrimidin-2-ylsulfanyl)-hexanoic acid ethyl ester

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 5.15 g / m-CPBA / CHCl3; CH2Cl2 / 20 h / -20 - 20 °C 2: 100 percent / Bu3SnH; AIBN / benzene / 1 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: m-CPBA 2: Bu3SnH, AIBN / benzene / 1 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate at 90 - 150℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With 2-nitrobenzeneseleninic acid; dihydrogen peroxide at 20℃; for 24h; | 76% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
106-97-8
n-butane

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
5870-68-8
ethyl 3-methylpentanoate

| Conditions | Yield |
|---|---|
| With C31H9AgBF27N6O at 0 - 40℃; under 190013 Torr; for 4h; Supercritical conditions; Sonication; Overall yield = 85 %; |

-
-
52089-55-1
2-hydroxyhexanoic acid ethyl ester

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide; Trimethylacetic acid In tetrahydrofuran at 20 - 22℃; for 2h; | 89 % Chromat. |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -20 - 20℃; | A n/a B 45% |
| In tetrahydrofuran at -20 - 20℃; | A n/a B 42% |

-
-
816-28-4
Ethyl 2-fluoro-2-hexenoate

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
17841-31-5
2-fluoro hexane-1-oate d'ethyle

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate Product distribution; Ambient temperature; other catalysts and other fluoroolefins; | A 1% B 99% |

| Conditions | Yield |
|---|---|
| With iodosylbenzene; potassium bromide In water at 20℃; for 12h; | 15 % Chromat. |
-
-
89984-56-5
methyl manganese chloride

-
-
72531-43-2
C9H16O4

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -20 - 20℃; | A 35% B n/a |

-
-
288400-65-7
2-(1-oxy-pyridine-2-sulfonyl)-hexanoic acid ethyl ester

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
175020-60-7
ethyl 2-(pyridin-2-ylsulfonyl)hexanoate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 1h; desulfonylation; Heating; | A 12% B 80% |
-
-
138286-76-7, 5445-29-4
ethyl-2-bromooctanoate

-
-
123-66-0
Ethyl hexanoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: 5.07 g / m-CPBA / CHCl3; CH2Cl2 / -20 - 20 °C 3: 91 percent / Bu3SnH; AIBN / toluene / 1 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 2: 5.07 g / m-CPBA / CHCl3; CH2Cl2 / -20 - 20 °C 3: 61 percent / m-CPBA / CHCl3; CH2Cl2 4: 12 percent / Bu3SnH; AIBN / benzene / 1 h / Heating View Scheme |
-
-
2396-83-0
ethyl hex-3-enoate

-
-
67-63-0
isopropyl alcohol

-
A
-
123-66-0
Ethyl hexanoate

-
B
-
2311-46-8
hexanoic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With 4,4'-di-tert-butylbiphenyl; lithium; nickel dichloride In tetrahydrofuran at 20 - 76℃; Inert atmosphere; chemoselective reaction; |


| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.5h; | 100% |
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Glovebox; Autoclave; | 93% |
| With ethanol; ruthenium(bis[2‐(ethylsulfanyl)ethyl]amine)(dichloro)(triphenylphosphine); potassium tert-butylate In toluene at 80℃; for 16h; Catalytic behavior; Reagent/catalyst; | 89% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B immobilized on palladium(0 and II)-stabilized UiO-66-NH2 metal organic framework modified with lauric acid nanocatalyst In toluene at 20℃; for 8h; Time; Sealed tube; Enzymatic reaction; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Amino-2-methyl-1-propanol With n-butyllithium; lanthanum(III) chloride In dichloromethane at 0℃; Metallation; after the reagent addition stirring for 15 min at this temperature under nitrogen, than warming to reflux; Stage #2: Ethyl hexanoate for 24h; Amidation; reflux; | 99% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.0833333h; microwave irradiation; | 98% |
-
-
123-66-0
Ethyl hexanoate

-
-
52598-04-6
1-<1,1-(2)H2>hexanol

| Conditions | Yield |
|---|---|
| With lithium aluminium deuteride In diethyl ether 1.) 0 deg C, 15 min, 2.) 0 deg C -> room temperature, 2 h; | 97% |
| With lithium aluminium deuteride In diethyl ether for 2h; Reduction; Heating; | 94% |
| With lithium aluminium deuteride In diethyl ether for 1h; Heating; | 89% |
| With lithium aluminium deuteride In diethyl ether at 0℃; | 83% |
-
-
123-66-0
Ethyl hexanoate

-
-
38002-45-8
3-bromo-1-(trimethylsilyl)-1-propyne

-
-
696622-96-5
1-Trimethylsilanyl-4-(3-trimethylsilanyl-prop-2-ynyl)-non-1-yn-4-ol

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-1-(trimethylsilyl)-1-propyne With chloro-trimethyl-silane; ethylene dibromide; zinc In tetrahydrofuran at 20℃; for 12h; Stage #2: Ethyl hexanoate In tetrahydrofuran at 20℃; Further stages.; | 96% |
-
-
123-66-0
Ethyl hexanoate

-
-
15821-77-9
Aluminium tris(phenylthiolate)

-
-
56974-15-3
S-phenyl hexanethioate

| Conditions | Yield |
|---|---|
| In benzene at 25℃; | 95% |
-
-
123-66-0
Ethyl hexanoate

-
-
18916-78-4
N-(pyridyl-3-imidoyl)guanidine

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: morpholine With diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 3h; Inert atmosphere; Stage #2: Ethyl hexanoate In tetrahydrofuran; hexane at 0℃; for 0.166667h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.0583333h; microwave irradiation; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: Ethyl hexanoate With morpholine; diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 3.16667h; Inert atmosphere; Stage #2: n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.166667h; Inert atmosphere; | 91% |
-
-
123-66-0
Ethyl hexanoate

-
-
1463-52-1
ethyl 2-(1-benzylpiperidin-4-ylidene)-2-cyanoacetate

-
-
1082204-05-4
C25H36N2O4

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; Michael addition; | 90% |
| Stage #1: Ethyl hexanoate With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; Cooling with acetone-dry ice; Stage #2: ethyl 2-(1-benzylpiperidin-4-ylidene)-2-cyanoacetate In tetrahydrofuran; hexane at -78℃; Cooling with acetone-dry ice; | 90% |
-
-
123-66-0
Ethyl hexanoate

-
-
95473-99-7
methyl 2-(3-methylthiophenylamino)-3-pyridine carboxylate

-
-
89108-93-0
3-(n-butyl)-4-hydroxy-1-(3-methylthiophenyl)-1,8-naphthyridin-2-(1H)-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate 1.) 0.25 h, 2.) heating, 4 h; | 89% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.0666667h; microwave irradiation; | 88% |

| Conditions | Yield |
|---|---|
| With 1-(3-sulfopropyl)pyridinium phosphotungstate In neat (no solvent) at 120℃; for 0.666667h; Microwave irradiation; | 88% |
-
-
123-66-0
Ethyl hexanoate

-
-
40067-82-1
N'-hydroxy-3-methylbenzimidamide

-
-
884625-45-0
5-pentyl-3-(m-tolyl)-1,2,4-oxadiazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) for 0.133333h; Microwave irradiation; | 85% |
-
-
123-66-0
Ethyl hexanoate

-
-
5033-28-3
4-chlorobenzamidoxime

-
-
884633-84-5
3-(4-chlorophenyl)-5-pentyl-1,2,4-oxadiazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) for 0.133333h; Microwave irradiation; | 85% |
-
-
123-66-0
Ethyl hexanoate

-
-
115891-56-0
methyl-3-(phenylamino)isonicotinate

-
-
115891-60-6
3-Butyl-4-hydroxy-1-phenyl-1H-[1,7]naphthyridin-2-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate 1.) 0.25 h, 2.) heating, 4 h; | 84% |
Ethyl caproate Consensus Reports
Ethyl caproate Specification
The CAS registry number of Ethyl caproate is 123-66-0. The IUPAC name is ethyl hexanoate. Its EINECS registry number is 204-640-3. In addition, the molecular formula is C8H16O2 and the molecular weight is 144.21. It is a kind of clear colourless liquid.
Physical properties about this chemical are: (1)ACD/LogP: 2.83; (2)ACD/LogD (pH 5.5): 2.83; (3)ACD/LogD (pH 7.4): 2.83; (4)ACD/BCF (pH 5.5): 83.88; (5)ACD/BCF (pH 7.4): 83.88; (6)ACD/KOC (pH 5.5): 829.01; (7)ACD/KOC (pH 7.4): 829.01; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 6; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.412; (12)Molar Refractivity: 40.88 cm3; (13)Molar Volume: 164 cm3; (14)Polarizability: 16.2 ×10-24cm3; (15)Surface Tension: 27.3 dyne/cm; (16)Density: 0.878 g/cm3; (17)Flash Point: 49.4 °C; (18)Enthalpy of Vaporization: 40.43 kJ/mol; (19)Boiling Point: 167.9 °C at 760 mmHg; (20)Vapour Pressure: 1.66 mmHg at 25°C.
Preparation of Ethyl caproate: it can be prepared by hexanoic acid and ethanol. This reaction will need reagent chlorotrimethylsilane and solvent diethyl ether. The reaction time is 1 hour by heating. The yield is about 87%.
.jpg)
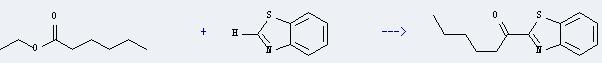
Uses of Ethyl caproate: it can be used as solvents, food additives, and intermediate in organic synthesis. And it can react with benzothiazole to get 1-benzothiazol-2-yl-hexan-1-one. This reaction will need reagent 1.6 M n-BuLi and solvents tetrahydrofuran and hexane. The reaction time is 1 hour at the temperature of -78 deg C. Then the reaction should react at room temperature for 30 minutes. The yield is about 63%.
When you are using this chemical, please be cautious about it as the following:
It is flammable and irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, keep away from sources of ignition and no smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)CCCCC
(2)InChI:InChI=1/C8H16O2/c1-3-5-6-7-8(9)10-4-2/h3-7H2,1-2H3
(3)InChIKey: SHZIWNPUGXLXDT-UHFFFAOYAA
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 123663-49-0
- 12366-52-8
- 1236699-92-5
- 123677-05-4
- 123-68-2
- 123689-95-2
- 123690-76-6
- 123690-78-8
- 123691-14-5
- 123-69-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View