-
Name
Ethyl caprylate
- EINECS 203-385-5
- CAS No. 106-32-1
- Article Data92
- CAS DataBase
- Density 0.868 g/cm3
- Solubility insoluble in water
- Melting Point -47 °C
- Formula C10H20O2
- Boiling Point 207.5 °C at 760 mmHg
- Molecular Weight 172.268
- Flash Point 75 °C
- Transport Information
- Appearance clear colourless liquid
- Safety 26-36
- Risk Codes 38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Octanoic acid, ethyl ester;Ethyl octanoate;Ethyl n-octanoate;Ethyl octoate;Ethyl octylate;
- PSA 26.30000
- LogP 2.91000
Synthetic route
-
-
2351-90-8, 7367-82-0, 42778-93-8
ethyl (2E)-oct-2-enoate

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride; cobalt acetylacetonate In tetrahydrofuran; hexane at -78 - 0℃; for 2h; | 99.5% |

| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) for 8h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With ethanol; (BQ‑NCOP)IrHCl; sodium t-butanolate at 60℃; for 8h; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With mesoporous MCM silicas at 60℃; for 6h; | 95% |
| With sulfuric acid at 25℃; for 0.5h; ultrasonic irradiation; | 95% |
| With TiO2-doped zirconia for 6h; Reflux; | 95.1% |

| Conditions | Yield |
|---|---|
| With triethylamine; dmap In dichloromethane at 0℃; for 0.5h; | 95% |
| With pentabutyl propyl guanidinium chloride; silica gel at 120℃; for 4h; | 95% |
| With dmap; triethylamine 1.) CH2Cl2, 0 deg C, 5 min, 2.) 0 deg C, 0.5 h; Yield given. Multistep reaction; |

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 10% palladium on activated carbon; hydrogen In ethanol at 20℃; for 3h; | 95% |
-
-
109-65-9
1-bromo-butane

-
-
151073-69-7
(4-ethoxy-4-oxobutyl)zinc(II) bromide

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride; tris(dibenzylideneacetone)dipalladium (0) In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 24h; Negishi cross-coupling; | 92% |
| With n-butylzinc bromide; tris(dibenzylideneacetone)dipalladium (0); 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 24h; Negishi cross-coupling; | 92% |
| With tris(dibenzylideneacetone)dipalladium (0); n-butylzinc bromide; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 24h; Negishi cross-coupling; | 92% |

| Conditions | Yield |
|---|---|
| With 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride; tris(dibenzylideneacetone)dipalladium (0) In tetrahydrofuran at 20℃; for 24h; Negishi cross-coupling; | 92% |
| tris(dibenzylideneacetone)dipalladium (0); 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 24h; Negishi cross-coupling; | 92% |
| Stage #1: n-butylzinc bromide With tris(dibenzylideneacetone)dipalladium (0); 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 1h; Stage #2: Ethyl 4-bromobutyrate In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; for 24h; Negishi cross-coupling; | 92% |
-
-
71478-41-6
(ethoxycarbonyl)octanoate

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine; dmap In dichloromethane for 0.25h; Ambient temperature; | 91% |
-
-
288400-58-8
ethyl 2-(pyrimidin-2-ylsulfonyl)octanoate

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 1h; desulfonylation; Heating; | 91% |

| Conditions | Yield |
|---|---|
| at 120℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| dilithium tetrachlorocuprate In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20℃; Alkylation; | 86% |
| With [((Me)NN2)NiCl] In tetrahydrofuran; ISOPROPYLAMIDE at -35 - 20℃; Inert atmosphere; | 85% |
| With N,N,N,N,-tetramethylethylenediamine; C34H46Cl4N10Ni2O2 In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 81% |

| Conditions | Yield |
|---|---|
| With 2-mesityl-2,5,6,7-tetrahydropyrrolo[2,1-c][1,2,4]triazol-4-ium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 60℃; for 24h; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: Octanal With hydroxylamine hydrochloride In dimethyl sulfoxide at 100℃; for 0.5h; Stage #2: ethanol With sulfuric acid In dimethyl sulfoxide at 130℃; for 2.5h; | 85% |
| With Mo(Oxo)/C at 90℃; for 2h; Catalytic behavior; | 50% |
| With manganese(IV) oxide; caesium carbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene; 1-butyl-3-methylimidazolium Tetrafluoroborate at 20℃; for 24h; Inert atmosphere; | 30% |
| (i) MeLi, THF, (ii) /BRN= 1744086/, (iii) O3; Multistep reaction; | |
| With oxygen at 100℃; under 3750.38 Torr; for 5h; Reagent/catalyst; Pressure; Autoclave; | 74 %Chromat. |

| Conditions | Yield |
|---|---|
| With 2-nitrobenzeneseleninic acid; dihydrogen peroxide at 20℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| In hexane; water for 6h; Solvent; Irradiation; | 77% |

| Conditions | Yield |
|---|---|
| With 3-(trifluoromethyl)styrene; bis(acetylacetonate)nickel(II) In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at -35℃; for 4h; Substitution; | 72% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 18 - 20℃; for 2.5h; | 70% |

| Conditions | Yield |
|---|---|
| In diethyl ether react. of Cu complex with organic electrophile at 0°C, 2 h; | 67% |
| In diethyl ether at 0℃; for 2h; | 67 % Chromat. |
-
-
64-17-5
ethanol

-
-
56519-71-2
1,3-propanediol dicaprylate

-
A
-
106-32-1
octanoic acid ethyl ester

-
B
-
624-13-5
propyl octanoate

| Conditions | Yield |
|---|---|
| With Mo(Oxo)/C at 90℃; under 10343.2 Torr; for 2h; Catalytic behavior; Inert atmosphere; | A 66% B 17% |


| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 3h; | 45% |
-
-
929-61-3
ethyl octyl ether

-
A
-
124-07-2
Octanoic acid

-
B
-
106-32-1
octanoic acid ethyl ester

-
C
-
1731-84-6
nonanoic acid methyl ester

-
D
-
2306-88-9
octyl octylate

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite In water at 20℃; for 16.25h; Oxidation; | A 31% B 7% C 13% D 44% |


| Conditions | Yield |
|---|---|
| With Mo(Oxo)/C at 90℃; under 10343.2 Torr; for 16h; Catalytic behavior; Inert atmosphere; | A 43% B 40% |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
110-54-3
hexane

-
A
-
106-32-1
octanoic acid ethyl ester

-
B
-
84612-77-1
3-ethyl-hexanoic acid, ethyl ester

-
C
-
37492-08-3
3-methyl-heptanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With C15HBBr3F18N6(1-)*Ag(1+)*C4H8O In water at 20℃; regioselective reaction; | A 23% B 10% C 30% D 7% E 9% |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
1188-92-7
Trihexylboran; Tri-n-hexylbor

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran |
-
-
592-41-6
1-hexene

-
-
5187-82-6
(ethoxycarbonylmethyl)dimethylsulfonium bromide

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| (i) BH3, (ii) /BRN= 4161472/, NaH, (iii) aq. H2O2, NaOH; Multistep reaction; |
-
-
13125-66-1
n-heptylmagnesium bromide

-
-
541-41-3
chloroformic acid ethyl ester

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| (i) MnI2, Et2O, (ii) /BRN= 385653/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| (i) I, (electrolysis), (ii) aq. NaOH, H2O2; Multistep reaction; |
-
-
64-17-5
ethanol

-
-
2264-29-1
2,2,2-trifluoroethyl octanoate

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In di-isopropyl ether at 25.5℃; Kinetics; immobilized lipase; various inhibitors and buffers; |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
110-54-3
hexane

-
A
-
106-32-1
octanoic acid ethyl ester

-
B
-
84612-77-1
3-ethyl-hexanoic acid, ethyl ester

-
C
-
37492-08-3
3-methyl-heptanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| RhTMPI at 60℃; Yield given. Yields of byproduct given; | |
| Rhpiv at 60℃; for 0.5h; Yield given. Yields of byproduct given; | |
| RhTPPI at 60℃; for 0.5h; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: octanoic acid ethyl ester With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -78℃; | 100% |
| With potassium tert-butylate In tetrahydrofuran for 0.0833333h; |
-
-
106-32-1
octanoic acid ethyl ester

-
-
78510-02-8
<1-2H2>-1-octanol

| Conditions | Yield |
|---|---|
| With lithium aluminium deuteride In tetrahydrofuran; diethyl ether at 0 - 20℃; for 5h; | 99% |
| With lithium aluminium deuteride In diethyl ether 1.) room temperature, 30 min 2.) reflux, 2 h; | 98% |
| With lithium aluminium deuteride |

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium hydroxide In toluene for 18h; Product distribution; Ambient temperature; | 98% |
| With AlBrCl3(1-)*C5H5N*H(1+) at 140℃; for 3h; | 95% |
| With Rhodococcus sp. LKE-028 esterase at 70℃; pH=11; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) at 64℃; for 8h; | 98% |
| With bromobenzene; hydrogen at 40℃; under 1500.15 Torr; for 3h; Autoclave; | 95% |
| With Dowex DR2030 ion exchange resin at 59.84℃; under 760.051 Torr; for 3h; |

| Conditions | Yield |
|---|---|
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Reagent/catalyst; Temperature; Pressure; Glovebox; Autoclave; | 96% |
| Stage #1: octanoic acid ethyl ester With C33H58FeN3PSi2; phenylsilane In toluene at 20℃; for 4h; Inert atmosphere; Glovebox; Green chemistry; Stage #2: With sodium hydroxide In toluene for 1h; Green chemistry; | 80% |
| With methanol; sodium tetrahydroborate; sodium ethanolate at 40℃; | 80% |
-
-
106-32-1
octanoic acid ethyl ester

-
-
108-91-8
cyclohexylamine

-
-
42577-04-8
N-(cyclohexyl)-n-octanoylamide

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.0583333h; microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: octanoic acid ethyl ester With sodium ethanolate; sodium carbonate In ethanol at 40℃; for 2.5h; Stage #2: With hydroxylamine In ethanol; water | 95.7% |
| With sodium sulfide; hydroxylamine hydrochloride; sodium hydroxide In ethanol at 20 - 45℃; for 3h; Temperature; Concentration; | 93.1% |
| With hydroxylamine hydrochloride; potassium hydroxide In ethanol; water at 5 - 55℃; for 3h; Concentration; Temperature; | 91.1% |
-
-
19721-22-3
3-sulfanylpropanol

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 31℃; for 168h; porcine pancreatic lipase; | 95.5% |

| Conditions | Yield |
|---|---|
| With ammonia at 40℃; for 24h; Temperature; Concentration; Enzymatic reaction; | 95% |
| With ammonia; water | |
| Multi-step reaction with 2 steps 1: Candida antarctica lipase B (Novozym 435); 1-butyl-3-methylimidazolium tetrafluoroborate; NH3 / 24 h / 40 °C 2: Candida antarctica lipase B (Novozym 435); 1-butyl-3-methylimidazolium tetrafluoroborate; NH3 / 96 h / 40 °C View Scheme |
-
-
106-32-1
octanoic acid ethyl ester

-
-
13307-76-1
[bis(phenylthio)methyl]lithium

-
-
75456-51-8
1,1-Bis-phenylsulfanyl-nonan-2-one

| Conditions | Yield |
|---|---|
| 95% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.05h; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; samarium diiodide In various solvent(s) at 20℃; for 0.5h; | 94% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 0.116667h; microwave irradiation; | 92% |
-
-
67-56-1
methanol

-
-
109-65-9
1-bromo-butane

-
-
106-32-1
octanoic acid ethyl ester

-
-
27610-92-0
2-butyloctanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: methanol; 1-bromo-butane; octanoic acid ethyl ester With sodium hydroxide at 20 - 55℃; for 7h; Large scale; Stage #2: With water; sodium carbonate at 70℃; for 6h; Large scale; | 91.43% |


| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) for 132h; Heating; | 90% |
-
-
14970-83-3
4-Mercapto-1-butanol

-
-
106-32-1
octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 30℃; for 48h; lipase from the yeast Candida cylindracea; | 88.4% |
-
-
106-32-1
octanoic acid ethyl ester

-
-
111198-02-8
1-(phenoxymethyl)-1H-benzo[d][1,2,3]triazole

-
-
189343-54-2
1-Benzotriazol-1-yl-1-phenoxy-nonan-2-one

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 20℃; for 12h; | 88% |

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)titanium dichloride; 4 A molecular sieve; magnesium; triethyl phosphite In tetrahydrofuran for 3h; Heating; | 88% |
-
-
106-32-1
octanoic acid ethyl ester

-
-
423-39-2
1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane

-
-
1262844-05-2
n-heptyl n-perfluorobutyl ketone

| Conditions | Yield |
|---|---|
| Stage #1: 1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane With ethylmagnesium bromide In diethyl ether at -70℃; for 1.5h; Stage #2: octanoic acid ethyl ester In diethyl ether at -70 - -60℃; for 16.5h; | 87% |
Ethyl caprylate Consensus Reports
Ethyl caprylate Specification
The CAS registry number of Ethyl caprylate is 106-32-1. Its EINECS registry number is 203-385-5. The IUPAC name is ethyl octanoate. In addition, the molecular formula is C10H20O2. What's more, it is a kind of clear colourless liquid and can be used as pharmaceutical intermediates and food flavors. And it should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 3.90; (2)ACD/LogD (pH 5.5): 3.9; (3)ACD/LogD (pH 7.4): 3.9; (4)ACD/BCF (pH 5.5): 538.64; (5)ACD/BCF (pH 7.4): 538.64; (6)ACD/KOC (pH 5.5): 3138.1; (7)ACD/KOC (pH 7.4): 3138.1; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 8; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.422; (12)Molar Refractivity: 50.15 cm3; (13)Molar Volume: 197 cm3; (14)Polarizability: 19.88 ×10-24cm3; (15)Surface Tension: 28.3 dyne/cm; (16)Density: 0.874 g/cm3; (17)Flash Point: 75 °C; (18)Enthalpy of Vaporization: 44.38 kJ/mol; (19)Boiling Point: 207.5 °C at 760 mmHg; (20)Vapour Pressure: 0.224 mmHg at 25°C.
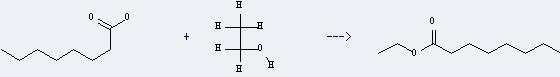
Preparation of Ethyl caprylate: it can be prepared by octanoic acid and ethanol through esterification reaction. Add octanoic acid, ethanol and a small amount concentrated sulfuric acid into the reactor. Then this reaction should reflux for 5 hours with haeting. And then after a series of neutralization by sodium hydroxide and distillation you can get the desired product.
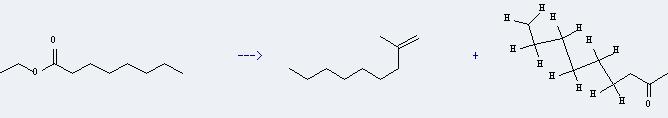
Uses of Ethyl caprylate: it can be used to get 2-methyl-non-1-ene and nonan-2-one. This reaction will need reagent trimethylstannylmethyllithium. The yield is about 58% at reaction temperature of -78 °C.
When you are using this chemical, please be cautious about it as the following:
It is irritating to skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)CCCCCCC
(2)InChI: InChI=1/C10H20O2/c1-3-5-6-7-8-9-10(11)12-4-2/h3-9H2,1-2H3
(3)InChIKey: YYZUSRORWSJGET-UHFFFAOYAU
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 25960mg/kg (25960mg/kg) | Food and Cosmetics Toxicology. Vol. 14, Pg. 763, 1976. |
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 106325-08-0
- 106-33-2
- 106-34-3
- 106-35-4
- 106356-53-0
- 106362-32-7
- 106-36-5
- 106366-30-7
- 1063734-78-0
- 106-37-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View