-
Name
Etoricoxib
- EINECS 682-421-5
- CAS No. 202409-33-4
- Article Data44
- CAS DataBase
- Density 1.298 g/cm3
- Solubility
- Melting Point 134-135 °C
- Formula C18H15ClN2O2S
- Boiling Point 510 °C at 760 mmHg
- Molecular Weight 358.848
- Flash Point 262.2 °C
- Transport Information
- Appearance off-white powder
- Safety 36/37-45
- Risk Codes 22-24
-
Molecular Structure
- Hazard Symbols T
- Synonyms 5-Chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]-2,3'-bipyridine;Algix;Arcoxia;Etobrix;Etocox;
- PSA 68.30000
- LogP 5.25670
Synthetic route
-
-
202409-40-3
etoricoxib hydrochloride

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane; water at 10 - 20℃; for 0.5h; Reagent/catalyst; Large scale; | 96.5% |

-
-
74-88-4
methyl iodide

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Stage #1: 4-(5-chloro-6'-methyl-[2,3'-bipyridin]-3-yl)benzenesulfinic acid With tetra-(n-butyl)ammonium iodide In tetrahydrofuran at 65℃; for 0.333333h; Stage #2: methyl iodide In tetrahydrofuran at 65℃; for 2h; Cooling with liquid nitrogen; | 95% |
-
-
292067-97-1
5-chloro-3-(4-methylsulphonyl)phenyl-2-(2-methyl-5-pyridinyl)pyridine

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With sodium molybdate; sulfuric acid; dihydrogen peroxide In methanol at 55℃; for 0.25h; | 94% |
| With oxygen; uranyl(VI) acetate dihydrate In o-xylene; water; acetonitrile at 20℃; under 760.051 Torr; Schlenk technique; Irradiation; | 91% |
| With diethylene glycol dibutyl ether; oxygen at 110℃; for 20h; Green chemistry; | 78% |

-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetonitrile at 80℃; for 8h; Concentration; Inert atmosphere; | 89% |
| With caesium carbonate In tetrahydrofuran at 20℃; for 3h; | 82% |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Stage #1: 1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 0.25h; Inert atmosphere; Stage #2: N-(2-chloro-3-(dimethylamino)allylidene)-N-methylmethanaminium hexafluorophosphate(V) With acetic acid In tetrahydrofuran at 20 - 25℃; for 2.5h; Inert atmosphere; Stage #3: With ammonium hydroxide In tetrahydrofuran at 60 - 65℃; for 1.5h; Inert atmosphere; | 84.4% |
| With methanesulfonic acid; ammonium acetate; propionic acid In toluene at 25 - 75℃; for 14 - 16h; Reflux; |
-
-
202409-86-7
2,5-dichloro-3-[4-(methylsulfonyl)phenyl]pyridine

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| palladium | 79% |

-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Stage #1: 1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone With potassium tert-butylate In tetrahydrofuran at 25 - 30℃; for 1h; Stage #2: 2-chloro-1,3-(bis-piperidinyl)trimethinium hexafluorophosphate In tetrahydrofuran at 30 - 55℃; Product distribution / selectivity; | 75.9% |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In 1,2-dichloro-ethane at 80 - 100℃; for 24h; | 73% |


-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In tetrahydrofuran at 60 - 65℃; Inert atmosphere; | 72.1% |
| With ammonium hydroxide In tetrahydrofuran for 5h; cyclocondensation; Heating; |

-
-
659742-21-9
(6-methylpyridin-3-yl)boronic acid

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium hydrogencarbonate In 1,4-dioxane; water at 80℃; for 8h; Suzuki Coupling; chemoselective reaction; | 71% |
-
-
3430-13-5
5-bromo-2-methylpyridine

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; caesium carbonate In 1,4-dioxane at 120℃; for 18h; Inert atmosphere; | 69% |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; propionic acid In toluene at 114℃; for 12h; Heating / reflux; | 65% |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
A
-
2457-47-8
3,5-dichloropyridine

-
B
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With ammonium acetate In propionic acid at 125℃; | A n/a B 62% C 15% |


-
-
659742-21-9
(6-methylpyridin-3-yl)boronic acid

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium hydrogencarbonate; tricyclohexylphosphine In 1,4-dioxane; water at 80℃; for 1.5h; Suzuki-Miyaura Coupling; Sealed tube; Inert atmosphere; Schlenk technique; | 60% |
| With tetrakis(triphenylphosphine) palladium(0); sodium hydrogencarbonate In 1,4-dioxane; water at 80℃; for 3h; Inert atmosphere; Schlenk technique; Sealed tube; | 52% |
-
-
99414-75-2
2,3-dichloroacrolein

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichloroacrolein; Lithium; (Z)-2-(4-methanesulfonyl-phenyl)-1-(6-methyl-pyridin-3-yl)-ethenolate In tetrahydrofuran Stage #2: With ammonia In tetrahydrofuran Heating; Further stages.; | 58% |
-
-
202409-81-2
2-bromo-5-chloro-3-(4-(methylsulfonyl)phenyl)pyridine

-
-
659742-21-9
(6-methylpyridin-3-yl)boronic acid

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); cesium fluoride In 1,4-dioxane for 20h; Concentration; Suzuki Coupling; Inert atmosphere; Reflux; | 55% |

-
-
149104-88-1
4-methanesulphonylphenylboronic acid

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate In 1,4-dioxane at 100℃; | 54% |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With ammonium acetate In propionic acid Heating; | 47% |

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In tetrahydrofuran for 3h; Heating; | 8.42 g |
-
-
53014-84-9
2-methyl-5-formylpyridine

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 46.5 g / isopropyl acetate / 60 °C 2: 46.5 g / isopropyl acetate / 60 - 65 °C 3: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 4: 24.91 g / aq. HCl / 25 - 45 °C 5: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 6: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 7: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: 46.5 g / isopropyl acetate / 60 °C 2: 46.5 g / isopropyl acetate / 60 - 65 °C 3: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 4: 24.91 g / aq. HCl / 25 - 45 °C 5: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 46.5 g / isopropyl acetate / 60 °C 2: 46.5 g / isopropyl acetate / 60 - 65 °C 3: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 4: 24.91 g / aq. HCl / 25 - 45 °C 5: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
5470-70-2
Methyl 6-methylnicotinate

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 3: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 4: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
90536-66-6
4-(methylsulfonyl)phenylacetic acid

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 3: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 4: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 58 percent / t-BuMgCl / tetrahydrofuran / 50 °C 2: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
221615-71-0
N-methoxy-N-methyl-6-methylnicotinamide

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 80 percent / tetrahydrofuran; toluene / 1 h / -10 °C 2: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 3: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 4: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 5: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / tetrahydrofuran; toluene / 1 h / -10 °C 2: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 3: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / tetrahydrofuran; toluene / 1 h / -10 °C 2: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 3: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
221615-72-1
1-(6-methyl-3-pyridinyl)-2-[4-(methyl sulfide)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 2: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 3: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 4: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 2: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 89 percent / sodium tungstate; aq. H2O2 / aq. H2SO4; methanol / 55 °C 2: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 2: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 3: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: t-BuOK / tetrahydrofuran / 0.75 h / 25 °C 2: TFA; AcOH / tetrahydrofuran / 0.75 h / 25 - 30 °C 3: aq. NH3 / tetrahydrofuran / 5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / tetrahydrofuran / 30 - 55 °C / Large scale 1.2: Large scale 2.1: sodium hydroxide; water / 25 - 35 °C View Scheme |

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 46.5 g / isopropyl acetate / 60 - 65 °C 2: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 3: 24.91 g / aq. HCl / 25 - 45 °C 4: t-BuOK / tetrahydrofuran / 0.75 h / 20 °C 5: AcOH; TFA / tetrahydrofuran / 0.75 h / 25 - 30 °C 6: 8.42 g / aq. NH4OH / tetrahydrofuran / 3 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 46.5 g / isopropyl acetate / 60 - 65 °C 2: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 3: 24.91 g / aq. HCl / 25 - 45 °C 4: 62 percent / NH4OAc / propionic acid / 125 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 46.5 g / isopropyl acetate / 60 - 65 °C 2: t-BuOK / propan-2-ol; tetrahydrofuran / 0.5 h / 25 °C 3: 24.91 g / aq. HCl / 25 - 45 °C 4: 47 percent / NH4OAc / propionic acid / Heating View Scheme |
-
-
3399-73-3
2-(1-cyclohexenyl)ethylamine

-
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With C47H70BrO4PPdSi; sodium t-butanolate In tetrahydrofuran at 20℃; for 1h; Schlenk technique; Inert atmosphere; Sealed tube; | 97% |
Etoricoxib Chemical Properties
Molecule structure of Etoricoxib (CAS NO.202409-33-4):
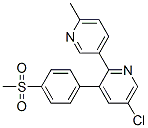
IUPAC Name: 5-Chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine
Molecular Weight: 358.8419 g/mol
Molecular Formula: C18H15ClN2O2S
Density: 1.298 g/cm3
Melting Point: 134-135 °C
Boiling Point: 510 °C at 760 mmHg
Flash Point: 262.2 °C
Index of Refraction: 1.6
Molar Refractivity: 94.65 cm3
Molar Volume: 276.4 cm3
Surface Tension: 49.6 dyne/cm
Enthalpy of Vaporization: 75.11 kJ/mol
Vapour Pressure: 5.17E-10 mmHg at 25 °C
XLogP3-AA: 3.3
H-Bond Acceptor: 4
Rotatable Bond Count: 3
Exact Mass: 358.054276
MonoIsotopic Mass: 358.054276
Topological Polar Surface Area: 59.9
Heavy Atom Count: 24
Canonical SMILES: CC1=NC=C(C=C1)C2=NC=C(C=C2C3=CC=C(C=C3)S(=O)(=O)C)Cl
InChI: InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3
InChIKey: MNJVRJDLRVPLFE-UHFFFAOYSA-N
Product Categories: Osteoarthritis and Rheumatoid Arthritis; Intermediates & Fine Chemicals; Pharmaceuticals
Etoricoxib Uses
Etoricoxib (CAS NO.202409-33-4) is a non-steroidal anti-inflammatory drug (NSAID). It is used for treating the diseases such as rheumatoid arthritis, psoriatic arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and gout.
Etoricoxib Specification
Etoricoxib (CAS NO.202409-33-4) is also named as 5-Chloro-6'-methyl-3-(p-(methylsulfonyl)phenyl)-2,3'-bipyridine ; Arcoxia ; Etoxib ; Etropain ; Kingcox ; L-791456 ; MK 0663 ; MK 663 ; Tauxib ; Torcoxia ; UNII-WRX4NFY03R ; 2,3'-Bipyridine, 5-chloro-6'-methyl-3-(4-(methylsulfonyl)phenyl)- . Etoricoxib (CAS NO.202409-33-4) is off-white powder.
Related Products
- Etoricoxib
- 20241-68-3
- 20241-76-3
- 20244-61-5
- 202460-48-8
- 202460-56-8
- 20246-33-7
- 202463-68-1
- 20246-53-1
- 202467-69-4
- 202468-69-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View