-
Name
Fmoc-N-trityl-L-asparagine
- EINECS
- CAS No. 132388-59-1
- Article Data7
- CAS DataBase
- Density 1.271 g/cm3
- Solubility
- Melting Point 201-204 °C(lit.)
- Formula C38H32N2O5
- Boiling Point 858.1 °C at 760 mmHg
- Molecular Weight 596.682
- Flash Point 472.8 °C
- Transport Information
- Appearance White to off white free flowing powder
- Safety 24/25
- Risk Codes 53
-
Molecular Structure
- Hazard Symbols
- Synonyms (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-(tritylcarbamoyl)propanoate;Nalpha-(9-Fluorenylmethoxycarbonyl)-Ngamma-trityl-L-asparagine;Fmoc-Asn(Trt)-OH;
- PSA 104.73000
- LogP 7.25850
Synthetic route
-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
132388-58-0
(S)-2-Amino-N-trityl-succinamic acid

-
-
132388-59-1
L-Asn(Trt)

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| With water In N,N-dimethyl-formamide for 24h; Product distribution; Ambient temperature; effect of water content on stability of various Fmoc-amino acid fluorides; |
-
-
76-84-6
triphenylmethyl alcohol

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / acetic anhydride, conc.H2SO4 / acetic acid / 1.25 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: acetic anhydride, conc.H2SO4 / acetic acid / 1 h / 50 °C 2: H2 / Pd-C View Scheme |
-
-
132388-57-9
N-benzyloxycarbonyl-N'-trityl-L-asparagine

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Pd-C View Scheme |
-
-
76-84-6
triphenylmethyl alcohol

-
-
71989-16-7
Fmoc-Asn-OH

-
-
107-06-2
1,2-dichloro-ethane

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic anhydride In tetrahydrofuran; methanol; di-isopropyl ether; water; acetic acid; ethyl acetate |


-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; di-isopropyl ether; water; ethyl acetate |


| Conditions | Yield |
|---|---|
| With water for 6h; Kinetics; |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (4-(hydroxymethyl)phenyl)diphenylphosphine oxide; L-Asn(Trt) With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0℃; for 0.166667h; Stage #2: With dmap In dichloromethane at 20℃; for 2h; | 99% |
-
-
132388-59-1
L-Asn(Trt)

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; | 98% |
| With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride | 98% |
-
-
71989-31-6
Fmoc-Pro-OH

-
-
71989-14-5
Fmoc-(tBu)Asp-OH

-
-
71989-26-9
Fmoc-Lys(tert-butoxycarbonyl)

-
-
132388-59-1
L-Asn(Trt)

-
-
77284-32-3
Fmoc-(S)-2-aminohexanoic acid

-
-
84624-17-9
N-(9-fluorenylmethoxycarbonyl)-D-valine

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-(tBu)Asp-OH With O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 0.833333h; 2-chlorotrityl resin; Automated synthesizer; Stage #2: With piperidine In N,N-dimethyl-formamide for 0.0833333h; 2-chlorotrityl resin; Automated synthesizer; Stage #3: Fmoc-Pro-OH; Fmoc-Lys(tert-butoxycarbonyl); L-Asn(Trt); Fmoc-(S)-2-aminohexanoic acid; N-(9-fluorenylmethoxycarbonyl)-D-valine Further stages; | 97% |


| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate In dichloromethane Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at -20 - -18℃; for 2h; | 96% |
-
-
132388-59-1
L-Asn(Trt)

-
-
619-45-4
4-methoxycarbonyl aniline

| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In dichloromethane at 0℃; for 2h; | 96% |
| With triethylamine; trichlorophosphate In dichloromethane at 0℃; for 2h; Inert atmosphere; | 13.97 g |

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Stage #1: HCl*H-Ser(tBu)-Phe-Arg(Pbf)-Tyr(tBu)-OKb, Kb=2,4-didocosyloxybenzyl; L-Asn(Trt) With 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; ethyl cyanoglyoxylate-2-oxime In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: With piperidine; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #3: With hydrogenchloride In tetrahydrofuran; water; N,N-dimethyl-formamide pH=Ca. 6; | 95.4% |
-
-
132388-59-1
L-Asn(Trt)

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
313944-83-1
(S)-Nα-(Fluoren-9-ylmethoxycarbonyl)-Nγ-tritylasparagine tert-butyldimethylsilyl ester

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.41667h; Esterification; | 95% |
-
-
35661-39-3
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine

-
-
132388-59-1
L-Asn(Trt)

-
-
146549-21-5
2-(9H-fluoren-9-ylmethoxycarbonylamino)-pent-4-enoic acid

-
-
170899-08-8
N-(tert-butoxycarbonyl)-D-allylglycine

-
-
170642-28-1
(R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)pent-4-enoic acid

-
-
936116-88-0
2-[2-(2-{2-[2-(2-tert-butoxycarbonylamino-pent-4-enoylamino)-propionylamino]-pent-4-enoylamino}-pent-4-enoylamino)-3-(trityl-carbamoyl)-propionylamino]-pent-4-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: C20H18NO4Pol With piperidine In 1-methyl-pyrrolidin-2-one not specified; Stage #2: L-Asn(Trt) With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one not specified; Stage #3: N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine; 2-(9H-fluoren-9-ylmethoxycarbonylamino)-pent-4-enoic acid; N-(tert-butoxycarbonyl)-D-allylglycine; (R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)pent-4-enoic acid Further stages; | 95% |

-
-
132388-59-1
L-Asn(Trt)

-
-
132388-58-0
(S)-2-Amino-N-trityl-succinamic acid

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide at 50℃; for 12h; | 95% |
-
-
623-04-1
4-aminobenzenemethanol

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| With N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline In tetrahydrofuran at 20℃; | 93% |
| Stage #1: L-Asn(Trt) With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide for 0.5h; Stage #2: 4-aminobenzenemethanol With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 3h; | 90% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane; N,N-dimethyl-formamide | |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 3.5h; | |
| With N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline In tetrahydrofuran at 20℃; | 19.2 g |
-
-
29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine

-
-
71989-31-6
Fmoc-Pro-OH

-
-
71989-14-5
Fmoc-(tBu)Asp-OH

-
-
71989-23-6
Fmoc-Ile-OH

-
-
71989-26-9
Fmoc-Lys(tert-butoxycarbonyl)

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-(tBu)Asp-OH With O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 0.833333h; 2-chlorotrityl resin; Automated synthesizer; Stage #2: With piperidine In N,N-dimethyl-formamide for 0.0833333h; 2-chlorotrityl resin; Automated synthesizer; Stage #3: N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-Pro-OH; Fmoc-Ile-OH; Fmoc-Lys(tert-butoxycarbonyl); L-Asn(Trt) Further stages; | 93% |

-
-
29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine

-
-
71989-14-5
Fmoc-(tBu)Asp-OH

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-(tBu)Asp-OH With N-ethyl-N,N-diisopropylamine In dichloromethane for 4h; Inert atmosphere; Stage #2: With piperidine In N,N-dimethyl-formamide for 0.35h; Stage #3: N-(fluoren-9-ylmethoxycarbonyl)glycine; C42H38N4O9; L-Asn(Trt); Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine Further stages; | 92% |

-
-
29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine

-
-
35661-40-6
N-Fmoc L-Phe

-
-
71989-14-5
Fmoc-(tBu)Asp-OH

-
-
132388-59-1
L-Asn(Trt)

| Conditions | Yield |
|---|---|
| Stage #1: HCl*H-Glu(OtBu)-OKb, Kb=2,4-didocosyloxybenzyl; N-Fmoc L-Phe With N-ethyl-N,N-diisopropylamine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With piperidine; benzotriazol-1-ol; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #3: N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-(tBu)Asp-OH; L-Asn(Trt) Further stages; | 90.8% |

-
-
68858-20-8
Fmoc-Val-OH

-
-
71989-33-8
Fmoc-Ser(tBu)-OH

-
-
71989-18-9
Fmoc-Glu(OtBu)-OH

-
-
108-24-7
acetic anhydride

-
-
132388-59-1
L-Asn(Trt)

-
-
1447762-81-3
Ac-S(tBu)E(OtBu)VN(trt)-OH

| Conditions | Yield |
|---|---|
| Stage #1: L-Asn(Trt) With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 3.25h; Stage #2: With piperidine In N,N-dimethyl-formamide Stage #3: Fmoc-Val-OH; Fmoc-Ser(tBu)-OH; Fmoc-Glu(OtBu)-OH; acetic anhydride Further stages; | 90% |


-
-
159610-93-2
Fmoc-O-methyl-L-serine

-
-
35661-40-6
N-Fmoc L-Phe

-
-
132388-59-1
L-Asn(Trt)

-
-
77128-72-4
N-(9-fluorenylmethoxycarbonyl)-O-methyl-L-tyrosine

| Conditions | Yield |
|---|---|
| Stage #1: (S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-6-(dimethylamino)hexanoic acid With N-ethyl-N,N-diisopropylamine In dichloromethane for 4h; Stage #2: With piperidine In N,N-dimethyl-formamide; isopropyl alcohol Stage #3: Fmoc-O-methyl-L-serine; N-Fmoc L-Phe; L-Asn(Trt); N-(9-fluorenylmethoxycarbonyl)-O-methyl-L-tyrosine Further stages; | 90% |

Fmoc-N-trityl-L-asparagine Chemical Properties
The Molecular Structure of Fmoc-Asn(Trt)-OH (132388-59-1):
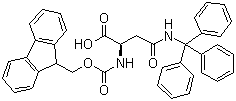
Molecular Formula: C38H32N2O5
Molecular Weight: 596.68
Nominal Mass: 596 Da
Average Mass: 596.6711 Da
Monoisotopic Mass: 596.231122 Da
Index of Refraction: 1.642
Molar Refractivity: 169.65 cm3
Molar Volume: 469.4 cm3
Surface Tension: 56.5 dyne/cm
Density: 1.271 g/cm3
Flash Point: 472.8 °C
Enthalpy of Vaporization: 130.66 kJ/mol
Boiling Point: 858.1 °C at 760 mmHg
Melting point: 205 oC
Vapour Pressure: 2.26E-31 mmHg at 25°C
Appearance: white crystal powder
Product Categories: Protected Amino Acids;Fluorenes, Flurenones;Amino Acids;Asparagine [Asn, N];Fmoc-Amino Acids and Derivatives;Amino Acids (N-Protected);Biochemistry;Fmoc-Amino Acids;Fmoc-Amino acid series
IUPAC Name: (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-4-oxo-4-(tritylamino)butanoate
Fmoc-N-trityl-L-asparagine Uses
Fmoc-Asn(Trt)-OH (132388-59-1) (CAS NO.132388-59-1) can be used as pharmaceutical intermediates.
Fmoc-N-trityl-L-asparagine Safety Profile
Safety Statements: 24/25
S24/25: Avoid contact with skin and eyes
WGK Germany: 2
F: 3-10
F3: Hygroscopic
F 10: Keep under argon
HazardClass: IRRITANT
Fmoc-N-trityl-L-asparagine Specification
Fmoc-Asn(Trt)-OH (132388-59-1) (CAS NO.132388-59-1) is also called as N-fmoc-l-asn(trt)-oh ; N-(9-fluorenyl methoxy carbonyl)-n-trityl-l-asparagine ; N-alpha-[(9h-fluoren-9-ylmethoxy)carbonyl]-n-gamma-trityl-l-asparagine ; N-alpha-(9-fluorenylmethyloxycarbonyl)-n-beta-trityl-l-asparagine ; N-alpha-9-fluorenylmethoxycarbonyl-n-omega-trityl-l-asparagine ; Nalpha-(9-fluorenylmethoxycarbonyl)-nr-trityl-l-asparagine ; N-alpha-(9-fluorenylmethoxycarbonyl)-n-beta-trityl-l-asparagine ; Nalpha-(9-Fluorenylmethoxycarbonyl)-Ngamma-trityl-L-asparagine ; Fmoc-N-trityl-L-asparagine .
Related Products
- Fmoc-N-trityl-L-asparagine
- 132388-60-4
- 132388-64-8
- 132388-65-9
- 132388-68-2
- 132388-69-3
- 13239-97-9
- 13240-18-1
- 1324-05-6
- 1324-11-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View