-
Name
Formestane
- EINECS 625-331-3
- CAS No. 566-48-3
- Article Data15
- CAS DataBase
- Density 1.19 g/cm3
- Solubility
- Melting Point 199-202 °C
- Formula C19H26O3
- Boiling Point 475.4 °C at 760 mmHg
- Molecular Weight 302.414
- Flash Point 255.4 °C
- Transport Information
- Appearance Needles
- Safety 53-36/37/39-45
- Risk Codes 60
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 4-HAD;4-Hydroxyandrost-4-ene-3,17-dione;4-Hydroxyandrostene-3,17-dione;4-Hydroxyandrostenedione;4-OHA;CRC 82/01;Lentaron;NSC 282175;
- PSA 54.37000
- LogP 3.97310
Synthetic route
-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel; acetic acid at 70℃; for 0.05h; | 86% |
| With boron trifluoride diethyl etherate In benzene for 19h; Ambient temperature; | 47% |
| With formic acid for 0.75h; Heating; | 45% |
| Multi-step reaction with 2 steps 1: 62 percent / aq. sodium hydroxide / 3 h / Heating 2: 53 percent / hydrochloric acid / dioxane / 24 h / Heating View Scheme |
-
-
180303-17-7
3-hydroxy-5α-androst-2-ene-4,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol for 1h; Ambient temperature; | 80% |
| With sodium In methanol at 20℃; for 1h; | 80% |
| With sodium methylate In methanol |

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol for 1h; Ambient temperature; | 80% |
-
-
434939-05-6
3β,4α-dihydroxy-5β-androstan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Stage #1: 3β,4α-dihydroxy-5β-androstan-17-one With dimethyl sulfoxide; triethylamine; trifluoroacetic anhydride In dichloromethane at -60℃; for 3h; Stage #2: With sodium In methanol at 20℃; for 3h; | 80% |
| Stage #1: 3β,4α-dihydroxy-5β-androstan-17-one With dimethyl sulfoxide; triethylamine; trifluoroacetic anhydride at -60℃; for 3.25h; Swern oxidation; Stage #2: With sodium methylate In methanol | |
| Multi-step reaction with 2 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 80 percent / Na / methanol / 1 h / 20 °C View Scheme |
-
-
434939-09-0
3-hydroxy-5β-androst-2-ene-4,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol at 20℃; for 1h; | 80% |
-
-
501433-34-7
5α/β-androst-3-en-17-one

-
A
-
63-05-8
Androstenedione

-
B
-
1229-12-5
androstane-3,17-dione

-
C
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multistep reaction; | A n/a B n/a C 54% |

-
-
20986-46-3
4-methoxy-4-androstene-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane for 24h; Heating; | 53% |
-
-
64-19-7
acetic acid

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
D
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.58 g D 0.7 g |

-
-
64-19-7
acetic acid

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4h,b) 4 deg C, 12 h; Further byproducts given; | A 52% B 0.58 g C 0.7 g D 3% |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
D
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.58 g D 0.7 g |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4h , b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.7 g D 3% |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a> RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 52% B 0.58 g C 0.7 g D 3% |


| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid for 18h; Ambient temperature; | 50% |
| With pyridine; hydrogen fluoride at 55℃; |
-
-
64-19-7
acetic acid

-
-
88509-24-4
4,5-epoxyandrost-9(11)-ene-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
140111-67-7
2α-hydroxyandrost-4,9(11)-diene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140111-66-6
2α-acetoxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |

-
-
88509-24-4
4,5-epoxyandrost-9(11)-ene-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
140111-67-7
2α-hydroxyandrost-4,9(11)-diene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140111-66-6
2α-acetoxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12h; | A 13.6% B n/a C 390 mg D 9% |
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |
| With sulfuric acid In acetic acid a) RT, 4h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sulfuric acid; dihydrogen peroxide 1a) 0 to 4 deg C, 30 min, MeOH, 1b) 5 to 7 deg C, 22 h, MeOH, 2) HOAc, RT, 45 min; Yield given. Multistep reaction; | |
| Multi-step reaction with 4 steps 1: 60 percent / Zn; AcOH / 0.25 h / 118 °C 2: 96 percent / aq. H2O2; HCO2H / 1 h / 20 °C 3: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 4: NaOMe / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme | |
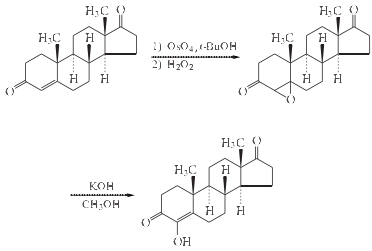
| Multi-step reaction with 2 steps 1: osmium tetroxide, 35percent H2O2 / 2-methyl-propan-2-ol / 72 h / Ambient temperature 2: potassium hydroxide / methanol / 0.17 h / 55 °C View Scheme | |
| Multi-step reaction with 2 steps 1: osmium(VIII) oxide; dihydrogen peroxide / tert-butyl alcohol / 72 h / 20 °C 2: potassium hydroxide / methanol / 0.17 h / 55 °C View Scheme |
-
-
110267-65-7
4ξ,5-dihydroxy-5ξ-androstane-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 55℃; for 0.166667h; Yield given; | |
| With potassium hydroxide In methanol at 55℃; for 0.166667h; | 0.452 g |
-
-
14935-81-0
5α-androst-3-en-17β-ol

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / aq. H2O2; HCO2H / 1 h / 20 °C 2: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 3: NaOMe / methanol View Scheme | |
| Multi-step reaction with 3 steps 1: 96 percent / H2O2; formic acid / CH2Cl2; H2O / 1 h / 20 °C 2: 98 percent / TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 3: 80 percent / Na / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) 30percent H2O2, 90percent aq. HCOOH 2.) aq. NaOH / 1.) r.t., 1 h, 2.) MeOH, 2: 1.) DMSO, TFAA 2.) NEt3 / 1.) CH2Cl2, -60 deg C, 10 min, 2.) -60 deg C, 15 min; -60 deg C to 5 deg C 3: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |
-
-
501433-34-7
5α/β-androst-3-en-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: aq. H2O2; HCO2H / CH2Cl2 / 6 h / 20 °C 2.1: 48 percent / HCO2H / 0.5 h / 20 °C 3.1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 3.2: NaOMe / methanol View Scheme |
-
-
37716-99-7
CGP 79318

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 2: NaOMe / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: 98 percent / TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 80 percent / Na / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) DMSO, TFAA 2.) NEt3 / 1.) CH2Cl2, -60 deg C, 10 min, 2.) -60 deg C, 15 min; -60 deg C to 5 deg C 2: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |
-
-
192192-77-1
3β,4β-epoxy-5β-androstan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 48 percent / HCO2H / 0.5 h / 20 °C 2.1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 2.2: NaOMe / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Na / methanol / 1 h / 20 °C 2: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme |
-
-
33386-50-4
3β,5β-dihydroxyandrostan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme | |
| Multi-step reaction with 4 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: Na / methanol / 1 h / 20 °C 3: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme |
-
-
434939-07-8
3α,4β-dihydroxy-5β-androstane-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 80 percent / Na / methanol / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: 1) DMSO, trifluoroacetic anhydride, 2) Et3N / 1) CH2Cl2, -60 deg C, 3 h, 2) CH3Cl2, -60 deg C, 15 min, -60 deg C to 5 deg C 3: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) DMSO, trifluoroacetic anhydride, 2) Et3N / 1) CH2Cl2, -60 deg C, 3 h, 2) CH3Cl2, -60 deg C, 15 min, -60 deg C to 5 deg C 2: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Jones reagent / acetone / -20 °C 2: osmium(VIII) oxide; dihydrogen peroxide / tert-butyl alcohol / 72 h / 20 °C 3: potassium hydroxide / methanol / 0.17 h / 55 °C View Scheme |
-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
-
75-36-5
acetyl chloride

-
-
61630-32-8
3,17-dioxoandrost-4-en-4-yl acetate

| Conditions | Yield |
|---|---|
| In pyridine for 3h; cooling; | 98% |
| With pyridine at 0 - 20℃; for 21.5h; | 72% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Substitution; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; water for 1h; Ambient temperature; | 79% |
-
-
108-24-7
acetic anhydride

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
-
61630-32-8
3,17-dioxoandrost-4-en-4-yl acetate

| Conditions | Yield |
|---|---|
| With pyridine Ambient temperature; | 67% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; water for 0.166667h; Ambient temperature; | 66% |
-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
-
134797-42-5
4-hydroxy-16-oximino-4-androsten-3,17-dione

| Conditions | Yield |
|---|---|
| With n-Amyl nitrite; potassium tert-butylate In tert-butyl alcohol for 2h; | 55% |
-
-
201230-82-2
carbon monoxide

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With di(rhodium)tetracarbonyl dichloride In 1,2-dichloro-benzene at 130℃; under 760.051 Torr; for 96h; Glovebox; | 55% |
| With di(rhodium)tetracarbonyl dichloride; tris(pentafluorophenyl)phosphine In 1,2-dichloro-benzene at 130℃; for 96h; | 55% |

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
-
98-88-4
benzoyl chloride

-
-
76942-05-7
Benzoic acid (8R,9S,10R,13S,14S)-10,13-dimethyl-3,17-dioxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-4-yl ester

| Conditions | Yield |
|---|---|
| With pyridine Ambient temperature; | 48% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 48h; Reflux; Dean-Stark; | 47% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 20℃; for 1h; Inert atmosphere; | 40% |
-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel; acetic acid at 70℃; for 0.05h; | 86% |
| With boron trifluoride diethyl etherate In benzene for 19h; Ambient temperature; | 47% |
| With formic acid for 0.75h; Heating; | 45% |
| Multi-step reaction with 2 steps 1: 62 percent / aq. sodium hydroxide / 3 h / Heating 2: 53 percent / hydrochloric acid / dioxane / 24 h / Heating View Scheme |
-
-
180303-17-7
3-hydroxy-5α-androst-2-ene-4,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol for 1h; Ambient temperature; | 80% |
| With sodium In methanol at 20℃; for 1h; | 80% |
| With sodium methylate In methanol |

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol for 1h; Ambient temperature; | 80% |
-
-
434939-05-6
3β,4α-dihydroxy-5β-androstan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Stage #1: 3β,4α-dihydroxy-5β-androstan-17-one With dimethyl sulfoxide; triethylamine; trifluoroacetic anhydride In dichloromethane at -60℃; for 3h; Stage #2: With sodium In methanol at 20℃; for 3h; | 80% |
| Stage #1: 3β,4α-dihydroxy-5β-androstan-17-one With dimethyl sulfoxide; triethylamine; trifluoroacetic anhydride at -60℃; for 3.25h; Swern oxidation; Stage #2: With sodium methylate In methanol | |
| Multi-step reaction with 2 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 80 percent / Na / methanol / 1 h / 20 °C View Scheme |
-
-
434939-09-0
3-hydroxy-5β-androst-2-ene-4,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With sodium In methanol at 20℃; for 1h; | 80% |
-
-
501433-34-7
5α/β-androst-3-en-17-one

-
A
-
63-05-8
Androstenedione

-
B
-
1229-12-5
androstane-3,17-dione

-
C
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multistep reaction; | A n/a B n/a C 54% |

-
-
20986-46-3
4-methoxy-4-androstene-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane for 24h; Heating; | 53% |
-
-
64-19-7
acetic acid

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
D
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.58 g D 0.7 g |

-
-
64-19-7
acetic acid

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4h,b) 4 deg C, 12 h; Further byproducts given; | A 52% B 0.58 g C 0.7 g D 3% |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
D
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.58 g D 0.7 g |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
2241-85-2
2α-acetoxyandrost-4-ene-3,17-dione

-
B
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4h , b) 4 deg C, 12 h; Further byproducts given; | A 23% B 52% C 0.7 g D 3% |

-
-
77057-73-9
4,5-epoxyandrostane-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
571-17-5
2alpha-Hydroxyandrost-4-ene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140223-19-4
4α-acetoxy-5α-hydroxyandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a> RT, 4 h, b) 4 deg C, 12 h; Further byproducts given; | A 52% B 0.58 g C 0.7 g D 3% |


| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid for 18h; Ambient temperature; | 50% |
| With pyridine; hydrogen fluoride at 55℃; |
-
-
64-19-7
acetic acid

-
-
88509-24-4
4,5-epoxyandrost-9(11)-ene-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
140111-67-7
2α-hydroxyandrost-4,9(11)-diene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140111-66-6
2α-acetoxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid a) RT, 4h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |

-
-
88509-24-4
4,5-epoxyandrost-9(11)-ene-3,17-dione

-
A
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

-
B
-
140111-67-7
2α-hydroxyandrost-4,9(11)-diene-3,17-dione

-
C
-
88509-26-6
4-hydroxyandrost-4,9(11)-diene-3,17-dione

-
D
-
140111-66-6
2α-acetoxyandrost-4,9(11)-diene-3,17-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12h; | A 13.6% B n/a C 390 mg D 9% |
| With sulfuric acid In acetic acid a) RT, 4 h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |
| With sulfuric acid In acetic acid a) RT, 4h, b) 4 deg C, 12 h; | A 13.6% B n/a C 390 mg D 9% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sulfuric acid; dihydrogen peroxide 1a) 0 to 4 deg C, 30 min, MeOH, 1b) 5 to 7 deg C, 22 h, MeOH, 2) HOAc, RT, 45 min; Yield given. Multistep reaction; | |
| Multi-step reaction with 4 steps 1: 60 percent / Zn; AcOH / 0.25 h / 118 °C 2: 96 percent / aq. H2O2; HCO2H / 1 h / 20 °C 3: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 4: NaOMe / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme | |
| Multi-step reaction with 2 steps 1: osmium tetroxide, 35percent H2O2 / 2-methyl-propan-2-ol / 72 h / Ambient temperature 2: potassium hydroxide / methanol / 0.17 h / 55 °C View Scheme | |
| Multi-step reaction with 2 steps 1: osmium(VIII) oxide; dihydrogen peroxide / tert-butyl alcohol / 72 h / 20 °C 2: potassium hydroxide / methanol / 0.17 h / 55 °C View Scheme |
-
-
110267-65-7
4ξ,5-dihydroxy-5ξ-androstane-3,17-dione

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 55℃; for 0.166667h; Yield given; | |
| With potassium hydroxide In methanol at 55℃; for 0.166667h; | 0.452 g |
-
-
14935-81-0
5α-androst-3-en-17β-ol

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / aq. H2O2; HCO2H / 1 h / 20 °C 2: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 3: NaOMe / methanol View Scheme | |
| Multi-step reaction with 3 steps 1: 96 percent / H2O2; formic acid / CH2Cl2; H2O / 1 h / 20 °C 2: 98 percent / TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 3: 80 percent / Na / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) 30percent H2O2, 90percent aq. HCOOH 2.) aq. NaOH / 1.) r.t., 1 h, 2.) MeOH, 2: 1.) DMSO, TFAA 2.) NEt3 / 1.) CH2Cl2, -60 deg C, 10 min, 2.) -60 deg C, 15 min; -60 deg C to 5 deg C 3: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |
-
-
501433-34-7
5α/β-androst-3-en-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: aq. H2O2; HCO2H / CH2Cl2 / 6 h / 20 °C 2.1: 48 percent / HCO2H / 0.5 h / 20 °C 3.1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 3.2: NaOMe / methanol View Scheme |
-
-
37716-99-7
CGP 79318

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 2: NaOMe / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: 98 percent / TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 80 percent / Na / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) DMSO, TFAA 2.) NEt3 / 1.) CH2Cl2, -60 deg C, 10 min, 2.) -60 deg C, 15 min; -60 deg C to 5 deg C 2: 80 percent / Na / methanol / 1 h / Ambient temperature View Scheme |
-
-
192192-77-1
3β,4β-epoxy-5β-androstan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 48 percent / HCO2H / 0.5 h / 20 °C 2.1: dimethyl sulfoxide; (CF3CO)2O; Et3N / 3.25 h / -60 °C 2.2: NaOMe / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Na / methanol / 1 h / 20 °C 2: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme |
-
-
33386-50-4
3β,5β-dihydroxyandrostan-17-one

-
-
566-48-3
4-hydroxyandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme | |
| Multi-step reaction with 4 steps 1: TFAA; Et3N; DMSO / CH2Cl2 / 3 h / -60 °C 2: Na / methanol / 1 h / 20 °C 3: 467 mg / Zn; glacial acetic acid / 0.17 h View Scheme |
Formestane Specification
The Formestane, with the CAS registry number 566-48-3, is also known as 4-Hydroxyandrostenedione. It belongs to the product categories of Pharmaceutical Raw Materials; Miscellaneous Biochemicals; Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals; Steroids. This chemical's molecular formula is C19H26O3 and molecular weight is 302.40794. Its IUPAC name is called (8R,9S,10R,13S,14S)-4-hydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,17-dione. it can be used as antitumor drug and aromatase inhibitor.
Physical properties of Formestane: (1)ACD/LogP: 2.66; (2)ACD/LogD (pH 5.5): 2.66; (3)ACD/LogD (pH 7.4): 2.66; (4)ACD/BCF (pH 5.5): 61.97; (5)ACD/BCF (pH 7.4): 61.22; (6)ACD/KOC (pH 5.5): 667.45; (7)ACD/KOC (pH 7.4): 659.45; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.569; (12)Molar Refractivity: 83.02 cm3; (13)Molar Volume: 253.1 cm3; (14)Surface Tension: 48.2 dyne/cm; (15)Density: 1.19 g/cm3; (16)Flash Point: 255.4 °C; (17)Enthalpy of Vaporization: 85.18 kJ/mol; (18)Boiling Point: 475.4 °C at 760 mmHg; (19)Vapour Pressure: 4.91E-11 mmHg at 25°C.
Preparation: this chemical can be prepared by 5-epoxy-5α-hsiung (steroid) alkyl-3,17-dione and methanol. This reaction will need CH3COOH, dichloromethane and hexane / ethyl acetate. The yield is about 47%.
When you are using this chemical, please be cautious about it as the following:
This chemical that at low levels can cause damage to health. It also may impair fertility. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC12CCC(=O)C(=C1CCC3C2CCC4(C3CCC4=O)C)O
(2)Isomeric SMILES: C[C@]12CCC(=O)C(=C1CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CCC4=O)C)O
(3)InChI: InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-13,22H,3-10H2,1-2H3/t11-,12-,13-,18+,19-/m0/s1
(4)InChIKey: OSVMTWJCGUFAOD-KZQROQTASA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View