-
Name
Glycidyl butyrate
- EINECS
- CAS No. 2461-40-7
- Article Data12
- CAS DataBase
- Density 1.069 g/cm3
- Solubility
- Melting Point
- Formula C7H12O3
- Boiling Point 196.258 °C at 760 mmHg
- Molecular Weight 144.17
- Flash Point 85 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Butanoicacid, oxiranylmethyl ester (9CI);Butyric acid, 2,3-epoxypropyl ester(6CI,7CI,8CI);(?à)-Glycidylbutyrate;2-Oxiranylmethyl butyrate;Glycidyl butanoate;Glycidyl butyrate;Glydicyl butyrate;NSC 83144;
- PSA 38.83000
- LogP 0.72850
Synthetic route

| Conditions | Yield |
|---|---|
| at 60℃; for 8h; | 95% |
| With 15-crown-5 In acetonitrile for 14h; Ambient temperature; | 92% |
| With tetrabutylammomium bromide In toluene for 5h; Solvent; Reflux; | 90% |
| With 1,4-dioxane at 200℃; | |
| With tetrabutylammomium bromide at 100℃; Kinetics; Temperature; |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In toluene for 5h; Solvent; Reflux; | 70% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 0℃; for 0.833333h; | |
| Stage #1: oxiranyl-methanol With trimethylamine In dichloromethane for 0.5h; Cooling with ice; Stage #2: butyryl chloride In dichloromethane at 20℃; for 3h; |
-
-
105-54-4
butanoic acid ethyl ester

-
-
107-18-6
allyl alcohol

-
A
-
2461-40-7
(+/-)-glycidyl butyrate

-
B
-
2051-78-7
allyl butyrate

-
C
-
556-52-5
oxiranyl-methanol

| Conditions | Yield |
|---|---|
| With Novozym 435; dihydrogen peroxide 1.) 15 min, 2.) 40 deg C, 16 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
107-18-6
allyl alcohol

-
A
-
2461-40-7
(+/-)-glycidyl butyrate

-
B
-
2051-78-7
allyl butyrate

-
C
-
556-52-5
oxiranyl-methanol

| Conditions | Yield |
|---|---|
| With Novozym 435; C3H7COOC2H5; dihydrogen peroxide 1.) 15 min, 2.) 40 deg C, 16 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
31027-31-3
4-isopropylphenylisocyanate

-
-
121373-21-5
<3-(4-isopropylphenyl)-2-oxo-5-oxazolidinyl>methyl butyrate

| Conditions | Yield |
|---|---|
| With Tributylphosphine oxide; lithium bromide In xylene for 2h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium (1S,4S)-bicyclo[2.2.1]hept-5-ene-2-carboxylate; palladium diacetate; XPhos In 1-methyl-pyrrolidin-2-one at 60℃; for 12h; Glovebox; Inert atmosphere; Sealed tube; | 97% |


| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium bicyclo[2.2.1]hept-2-ene-5-carboxylate; XPhos In 1-methyl-pyrrolidin-2-one at 60℃; for 12h; | 97% |


| Conditions | Yield |
|---|---|
| With 18-crown-6 ether at 120℃; under 39003.9 Torr; for 16h; Autoclave; | 93% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
75-86-5
2-hydroxy-2-methylpropanenitrile

-
-
142944-71-6
Butyric acid 3-cyano-2-hydroxy-propyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 3.5h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; XPhos In 1-methyl-pyrrolidin-2-one at 80℃; for 24h; Inert atmosphere; Sealed tube; | 91% |
| With palladium diacetate; potassium bicyclo[2.2.1]hept-2-ene-5-carboxylate; XPhos In 1-methyl-pyrrolidin-2-one at 80℃; for 24h; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: (+/-)-glycidyl butyrate With {Eu[N(SiMe3)2](μ-O:κ2-C6H5C(O)NC6H3(iPr)2)(THF)}2 at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: carbon disulfide at 50℃; for 6h; Inert atmosphere; | 91% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
615-37-2
ortho-methylphenyl iodide

-
-
89343-06-6
tris-iso-propylsilyl acetylene

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; norborn-2-ene; potassium acetate; palladium diacetate; XPhos at 60℃; for 24h; Glovebox; Inert atmosphere; Sealed tube; regioselective reaction; | 83% |


| Conditions | Yield |
|---|---|
| With 1,1-dimethyl-3,3-diethylguanidinecarbonylcobalt at 80℃; under 45004.5 Torr; for 24h; Autoclave; | 79% |
| With 1,1-dimethyl-3,3-diethylguanidinecarbonylcobalt at 80℃; under 45004.5 Torr; for 24h; Autoclave; Schlenk technique; | 79% |


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide; toluene at -30 - 20℃; | 75% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
790703-40-1
(4-benzyloxy-3-fluorophenyl)carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (4-benzyloxy-3-fluorophenyl)carbamic acid benzyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.25h; Stage #2: (+/-)-glycidyl butyrate In tetrahydrofuran; hexane at -78 - 20℃; | 65.5% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
125188-61-6
Butyric acid thiiranylmethyl ester

| Conditions | Yield |
|---|---|
| With thiourea | 60% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
5309-42-2
1-sn-monobutyroylglycerol

-
B
-
60456-26-0
(R)-glycidyl butyrate

-
C
-
126254-87-3
(S)-2,3-dihydroxypropyl butyrate

| Conditions | Yield |
|---|---|
| With [(S,S)-N,N’-bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]cobalt(II); air; acetic acid In tetrahydrofuran at 0 - 20℃; | A n/a B 46% C n/a |

-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
5309-42-2
1-sn-monobutyroylglycerol

-
B
-
126254-87-3
(S)-2,3-dihydroxypropyl butyrate

-
C
-
65031-96-1
(S)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| With (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II); air; acetic acid In tetrahydrofuran at 0 - 20℃; | A n/a B n/a C 46% |
| With water; chiral ((substituted salen)Co)2-InCl3 at 20℃; for 3h; | A n/a B n/a C 45% |
| With water; (R,R)-[Co(salen)]2*GaCl3 at 20℃; for 4h; | A n/a B n/a C 43% |
| With water; chiral salen-based cobalt(II) complex at 20℃; for 4h; | A n/a B n/a C 40% |

-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
5309-42-2
1-sn-monobutyroylglycerol

-
B
-
65031-96-1
(S)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| With (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II); air; acetic acid In tetrahydrofuran at 0 - 20℃; for 16h; | A n/a B 46% |
| With 5CF3O3S(1-)*2Co(3+)*Y(3+)*2C36H52N2O2(2-); water In neat (no solvent) at 20℃; for 4h; Overall yield = 44 %; enantioselective reaction; | A n/a B n/a |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
60456-26-0
(R)-glycidyl butyrate

-
B
-
126254-87-3
(S)-2,3-dihydroxypropyl butyrate

| Conditions | Yield |
|---|---|
| With [(S,S)-N,N’-bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]cobalt(II); air; acetic acid In tetrahydrofuran at 0 - 20℃; | A 46% B n/a |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
2150-89-2
(3-chlorophenyl) carbamic acid ethyl ester

-
-
42902-30-7
3-(3-chlorophenyl)-5-(hydroxymethyl)oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -78 - 20℃; | 46% |

| Conditions | Yield |
|---|---|
| With [methanesulfonato(2-dicyclohexylphosphino-2',6'-di-isopropoxy-1,1'-biphenyl)(2'-methylamino-1,1'-biphenyl-2-yl)palladium(II)]; C11H16O2; sodium acetate In N,N-dimethyl-formamide at 120℃; for 24h; Inert atmosphere; Glovebox; | 46% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
60456-26-0
(R)-glycidyl butyrate

-
B
-
57044-25-4
(R)-oxiranemethanol

| Conditions | Yield |
|---|---|
| With sodium phosphate buffer; 25 kDa lipase-like enzyme immobilized on DEAE-Sepharose In 1,4-dioxane; water at 25℃; for 10h; pH=7.00; | A n/a B 45% |
| With thermomyces lanuginosa In 1,4-dioxane; aq. phosphate buffer at 30℃; for 2h; pH=7; Temperature; Resolution of racemate; Enzymatic reaction; | A n/a B n/a |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
890051-54-4
butyric acid-3-chloro-(S)-2-hydroxy-propyl ester

-
B
-
890051-57-7
butyric acid-3-chloro-(R)-2-hydroxy-propyl ester

-
C
-
60456-26-0
(R)-glycidyl butyrate

-
D
-
65031-96-1
(S)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; (R,R)-[Co(salen)]2*GaCl3 In diethyl ether at 0 - 4℃; for 4h; | A 45% B n/a C n/a D 43% |

-
-
2461-40-7
(+/-)-glycidyl butyrate

-
-
65031-96-1
(S)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 44% |
-
-
2461-40-7
(+/-)-glycidyl butyrate

-
A
-
60456-26-0
(R)-glycidyl butyrate

-
B
-
126254-87-3
(S)-2,3-dihydroxypropyl butyrate

-
C
-
65031-96-1
(S)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| With (acetao)(aqua)(S,S)-N,N′-bis(3,5-di-tert-butylsalicylidene-1,2-cyclohexanediamino)-cobalt(III); water In tetrahydrofuran at 0℃; for 12h; Jacobsen rearrangement; optical yield given as %ee; | A 40% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In tetrahydrofuran for 0.333333h; Heating; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With tert-butoxyl radical In various solvent(s) |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 100℃; for 5h; |
-
-
2461-40-7
(+/-)-glycidyl butyrate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; trimethylsilylazide; lithium isopropoxide; ytterbium(III) triflate 1) THF, r.t., 17 h, 2) THF; Yield given. Multistep reaction. Yields of byproduct given; |
Glycidyl butyrate Chemical Properties
Product Name: Glycidyl butyrate (2461-40-7)
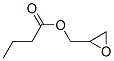
Molecular Formula: C7H12O3
Molecular Weight: 144.17g/mol
Mol File: 2461-40-7.mol
Boiling point: 196.3 °C at 760 mmHg
Flash Point: 85 °C
Density: 1.069 g/cm3
Product Categories: API intermediates
Synonyms of Glycidyl butyrate (2461-40-7): glycidyl butyrate ; (r)-2,3-epoxypropyl butyrate ; 2,3-epoxypropyl ester butyric acid .
Glycidyl butyrate Uses
Glycidyl butyrate (2461-40-7) is widely used in chiral drugs, pesticides, spices chiral synthesis.Ink. Resin. Acrylic paint. Adhesives. Latex. Plastic alloy. Photosensitive materials, organic synthesis, polymer synthesis of modified.
Glycidyl butyrate Specification
Glycidyl butyrate (2461-40-7) is a colorless and transparent liquid, insoluble in water, almost insoluble in all organic solvents, on the skin and mucous membrane irritant, almost non-toxic. Because of its carbon-carbon molecule that contains both the double bond, but also contains epoxy groups, both to free radical-type reaction, but also for ion-type reaction, therefore, has a very high response flexibility to separate the different reactions. widely used in medicine, photosensitive materials, organic synthesis and polymer-modified and other fields. derived products have excellent anti-UV, water-resistant heat-resistant characteristics, is an important fine chemical raw material.
Related Products
- Glycidyl 4-nonylphenyl ether
- Glycidyl acrylate
- Glycidyl butyrate
- Glycidyl hexyl ether
- Glycidyl methacrylate
- Glycidyl methyl ether
- Glycidyl oleate
- Glycidyl phenyl ether
- Glycidyl p-tolyl ether
- Glycidylmethylaniline
- 2461-42-9
- 2461-43-0
- 2461-46-3
- 24615-84-7
- 246159-33-1
- 246166-33-6
- 2461-80-5
- 24619-05-4
- 24621-61-2
- 24621-73-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View