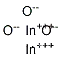-
Name
Indium oxide
- EINECS 215-193-9
- CAS No. 1312-43-2
- Density 7.18 g/mL at 25 °C(lit.)
- Solubility <0.01 mm Hg ( 25 °C) in water
- Melting Point 2000 °C
- Formula In2O3
- Boiling Point 850oC
- Molecular Weight 277.64
- Flash Point 13°(55°F)
- Transport Information UN 1993
- Appearance light green powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Diindiumtrioxide;EP-ITO-DL 1;India;Indium oxide (2:3);Indiumsesquioxide;Indium trioxide;Indium(3+) oxide;Indium(III) oxide;Mesatron;Trioxodiindium;
- PSA 43.37000
- LogP -0.30600
Indium oxide Consensus Reports
Indium oxide Specification
The Indium oxide, with the cas registry number 1312-43-2, has the IUPAC name of oxo(oxoindiganyloxy)indigane. This is a kind of white to light yellow powder and is stable but incompatible with acids. Besides, its product categories are various, including Inorganics; 49: In; IndiumMaterials Science; Nanomaterials; Nanoparticles: Oxides, Nitrides, and Other CeramicsNanomaterials; Nanopowders and Nanoparticle Dispersions; Catalysis and Inorganic Chemistry; Chemical Synthesis; Indium; IndiumMetal and Ceramic Science; Oxides.
The characteristics of this chemical are as follows: (1)H-Bond Acceptor: 3; (2)Exact Mass: 277.7925; (3)MonoIsotopic Mass: 277.7925; (4)Topological Polar Surface Area: 43.4; (5)Heavy Atom Count: 5; (6)Complexity: 34.2; (7)Covalently-Bonded Unit Count: 1; (8)Melting point: 2000°C; (9)Density: 7.18 g/mL at 25 °C(lit.); (10)Vapor pressure: <0.01 mm Hg ( 25 °C).
When you are using this chemical, you should be very cautious. For being a kind of irritant chemical, it is irritating to eyes, respiratory system and skin and may cause inflammation to the skin or other mucous membranes. Therefore, you should wear suitable protective clothing and if in case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
As to its usage, it is widely applied in many ways. This chemical could be used as the spectrum pure reagent and the material of electronic component; It is also used in some types of batteries, thin film infrared reflectors transparent for visible light (hot mirrors), some optical coatings, and some antistatic coatings,etc.
Additionally, you could convert the following datas into the molecular structure:
(1)Canonical SMILES: O=[In]O[In]=O
(2)InChI: InChI=1S/2In.3O
(3)InChIKey: SHTGRZNPWBITMM-UHFFFAOYSA-N
Below are the toxicity information of this chemical:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD | oral | > 10gm/kg (10000mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 29(12), Pg. 38, 1985. | |
| mouse | LDLo | intraperitoneal | 5gm/kg (5000mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 30(10), Pg. 28, 1965. | |
| mouse | LDLo | oral | 10gm/kg (10000mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 30(10), Pg. 28, 1965. | |
| rat | LD | oral | > 10gm/kg (10000mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 29(12), Pg. 38, 1985. |
Related Products
- Indium
- Indium acetate
- Indium acetylacetonate
- Indium antimonide (InSb)
- Indium arsenide
- Indium bromide (InBr<sub>3</sub>)
- Indium chloride
- Indium chloride(InCl3), tetrahydrate (8CI,9CI)
- INDIUM CITRATE
- Indium dicyanide
- 1312-45-4
- 13125-66-1
- 131256-74-1
- 13125-74-1
- 13126-12-0
- 13126-70-0
- 131-27-1
- 13127-18-9
- 13127-28-1
- 1312-73-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View