-
Name
KANAMYCIN
- EINECS 200-411-7
- CAS No. 59-01-8
- Article Data14
- CAS DataBase
- Density 1.62g/cm3
- Solubility
- Melting Point
- Formula C18H36 N4 O11
- Boiling Point 809.5°Cat760mmHg
- Molecular Weight 484.504
- Flash Point 443.4°C
- Transport Information
- Appearance
- Safety Poison by intravenous and intramuscular routes. Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms KanamycinA (7CI);4,6-Diamino-2-hydroxy-1,3-cyclohexane 3,6'-diamino-3,6'-dideoxydi-a-D-glucoside;KM;Kanacin;Kanamycin;
- PSA 282.61000
- LogP -4.49020
Synthetic route

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With Amberlite IRA-400-OH In water | 91% |
| With Amberlite IRA-400 -OH In water | |
| With Amberlite-IRA 400 (OH-) |

| Conditions | Yield |
|---|---|
| With hydrogen; acetic acid; palladium In water under 760 Torr; for 2h; Ambient temperature; | A 4.7% B 83% |
-
-
98084-09-4
6'-N-hydroxykanamicyn A (sulfate)

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; platinum(IV) oxide In water under 2660 Torr; for 2h; Ambient temperature; Yield given; |
-
-
80860-34-0
di-tert-butyl ((1S,3R,4S,5R,6R)-4-(((2S,3R,4S,5S,6R)-4-((tert-butoxycarbonyl)amino)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6-(((2R,3R,4S,5S,6R)-6-(((tert-butoxycarbonyl)amino)methyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-5-hydroxycyclohexane-1,3-diyl)dicarbamate

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| tetrafluoroboric acid at 20℃; for 0.1h; |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen; nickel under 2280 Torr; for 5h; Ambient temperature; |
-
-
74256-73-8
3,6'-bis(N-benzyloxycarbonyl)-3"-N-(trifluoroacetyl)kanamycin A

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 86 percent / sodium tungstate dihydrate, aq. H2O2 / various solvent(s) / Ambient temperature 2: aq. NH4OH / various solvent(s) / Ambient temperature 3: 4.7 percent / H2, AcOH / Pd black / H2O; various solvent(s) / 2 h / 760 Torr / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: 86 percent / sodium tungstate dihydrate, aq. H2O2 / various solvent(s) / Ambient temperature 2: aq. NH4OH / various solvent(s) / Ambient temperature 3: 83 percent / H2, AcOH / Pd black / H2O; various solvent(s) / 2 h / 760 Torr / Ambient temperature 4: H2, aq. NH4OH / Raney Ni / 5 h / 2280 Torr / Ambient temperature View Scheme |

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 83 percent / H2, AcOH / Pd black / H2O; various solvent(s) / 2 h / 760 Torr / Ambient temperature 2: H2, aq. NH4OH / Raney Ni / 5 h / 2280 Torr / Ambient temperature View Scheme |

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NH4OH / various solvent(s) / Ambient temperature 2: 4.7 percent / H2, AcOH / Pd black / H2O; various solvent(s) / 2 h / 760 Torr / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: aq. NH4OH / various solvent(s) / Ambient temperature 2: 83 percent / H2, AcOH / Pd black / H2O; various solvent(s) / 2 h / 760 Torr / Ambient temperature 3: H2, aq. NH4OH / Raney Ni / 5 h / 2280 Torr / Ambient temperature View Scheme |

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With His-tagged NADPH-dependent reductase; potassium chloride; glycerol; NADPH at 28℃; pH=7.5; Tris-HCl buffer; Enzymatic reaction; stereoselective reaction; |

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 20h; Reagent/catalyst; Solvent; Temperature; |
-
-
124068-97-9
4‐nitrophenyl N‐benzylcarbamate

-
-
59-01-8
kanamycin A

-
-
1054616-53-3
1-benzyl-3-{5-(3-benzyl-ureido)-4-[4-(3-benzyl-ureido)-3,5-dihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy]-2-[6-(3-benzyl-ureidomethyl)-3,4,5-trihydroxy-tetrahydro-pyran-2-yloxy]-3-hydroxy-cyclohexyl}-urea

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water at 20℃; for 3h; | 93% |
-
-
59-01-8
kanamycin A

-
-
207857-15-6
1,3-di(tert-butyloxycarbonyl)-2-(trifluoromethylsulfonyl)guanidine

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water at 20℃; | 91% |
-
-
59-01-8
kanamycin A

-
-
207857-15-6
1,3-di(tert-butyloxycarbonyl)-2-(trifluoromethylsulfonyl)guanidine

-
-
290360-47-3
C62H108N12O27

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water at 20℃; for 72h; | 91% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
59-01-8
kanamycin A

-
-
80860-34-0
di-tert-butyl ((1S,3R,4S,5R,6R)-4-(((2S,3R,4S,5S,6R)-4-((tert-butoxycarbonyl)amino)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6-(((2R,3R,4S,5S,6R)-6-(((tert-butoxycarbonyl)amino)methyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-5-hydroxycyclohexane-1,3-diyl)dicarbamate

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 60℃; for 5h; | 91% |
| With sodium carbonate In methanol; water at 20℃; for 4h; | 90% |
| In water; dimethyl sulfoxide at 70℃; for 20h; | 84% |
| In water; dimethyl sulfoxide at 70℃; for 20h; | 84% |
| With triethylamine In water; N,N-dimethyl-formamide at 60℃; for 6h; Inert atmosphere; | 76% |
-
-
1538-75-6
2,2-dimethylpropanoic anhydride

-
-
59-01-8
kanamycin A

-
-
80860-34-0
di-tert-butyl ((1S,3R,4S,5R,6R)-4-(((2S,3R,4S,5S,6R)-4-((tert-butoxycarbonyl)amino)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6-(((2R,3R,4S,5S,6R)-6-(((tert-butoxycarbonyl)amino)methyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-5-hydroxycyclohexane-1,3-diyl)dicarbamate

| Conditions | Yield |
|---|---|
| In methanol; water | 90% |
-
-
501-53-1
benzyl chloroformate

-
-
59-01-8
kanamycin A

-
-
5605-66-3
1,3,6',2''-tetra-N-benzyloxycarbonyl-kanamycin A

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetone at 20℃; for 18h; | 84% |
| With sodium carbonate In water Yield given; |

| Conditions | Yield |
|---|---|
| Stage #1: kanamycin A With chloro-trimethyl-silane; 1,1,1,3,3,3-hexamethyl-disilazane In acetonitrile Reflux; Stage #2: C16H20N2O4S2 In acetone; acetonitrile at 0 - 5℃; for 1h; Stage #3: With hydrogenchloride In water; acetone; acetonitrile for 1h; pH=2 - 3; Solvent; Reagent/catalyst; Temperature; | 83% |
-
-
1608113-35-4
C31H30N3O12P

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water at 0 - 20℃; for 16h; | 82% |
-
-
23776-85-4
N-(phenylacetyl)oxysuccinimide

-
-
59-01-8
kanamycin A

-
-
1352640-83-5
tetra N-phenylacetyl-kanamycin A

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; N,N-dimethyl-formamide at 20℃; | 81% |
-
-
152120-54-2, 862686-58-6, 1143572-00-2
N,N'-bis( tert-butoxycarbonyl)-1H-pyrazole-1-carboxamidine

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane; water at 20℃; for 5h; | 78% |

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 50℃; for 72h; | 77% |
-
-
59-01-8
kanamycin A

-
-
80860-34-0
di-tert-butyl ((1S,3R,4S,5R,6R)-4-(((2S,3R,4S,5S,6R)-4-((tert-butoxycarbonyl)amino)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6-(((2R,3R,4S,5S,6R)-6-(((tert-butoxycarbonyl)amino)methyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-5-hydroxycyclohexane-1,3-diyl)dicarbamate

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water for 24h; Ambient temperature; | 75% |
-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide Ambient temperature; | 70% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol; water at 0℃; | 68% |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
59-01-8
kanamycin A

-
-
5605-66-3
1,3,6',2''-tetra-N-benzyloxycarbonyl-kanamycin A

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; N,N-dimethyl-formamide at 20℃; | 66% |
-
-
93801-72-0
p-methoxybenzyl-S-(4,6-dimethylpyrimidine-2-yl)thiocarbonate

-
-
59-01-8
kanamycin A

-
-
93801-63-9
3,6'-di-N-p-methoxybenzyloxycarbonyl kanamycin A

| Conditions | Yield |
|---|---|
| With nickel diacetate In dimethyl sulfoxide for 0.5h; | 65% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; water for 20h; | 55% |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
59-01-8
kanamycin A

-
-
66567-24-6
3,6'-bis-N-benzyloxycarbonylkanamycin A

| Conditions | Yield |
|---|---|
| Stage #1: kanamycin A With zinc diacetate In dimethyl sulfoxide Inert atmosphere; Stage #2: N-(Benzyloxycarbonyloxy)succinimide In dimethyl sulfoxide Inert atmosphere; | 50% |
| With zinc diacetate In dimethyl sulfoxide Inert atmosphere; | 50% |
| Stage #1: kanamycin A With zinc diacetate In dimethyl sulfoxide at 20℃; for 12h; Stage #2: N-(Benzyloxycarbonyloxy)succinimide In dimethyl sulfoxide for 2h; |
-
-
59-01-8
kanamycin A

-
-
17029-36-6
Kanamycin A-3'-phosphate

| Conditions | Yield |
|---|---|
| With Tmed buffer; magnesium acetate In water for 12h; aminoglycoside phosphotransferase; | 44% |
-
-
139705-38-7
acetone O-<(vinyloxy)carbonyl>oxime

-
-
59-01-8
kanamycin A

-
-
791064-46-5
{4-(3,5-dihydroxy-6-hydroxymethyl-4-vinyloxycarbonylamino-tetrahydro-pyran-2-yloxy)-3-hydroxy-2-[3,4,5-trihydroxy-6-(vinyloxycarbonylamino-methyl)-tetrahydro-pyran-2-yloxy]-5-vinyloxycarbonylamino-cyclohexyl}-carbamic acid vinyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In phosphate buffer at 0℃; for 5h; pH=8.0; | 37% |

| Conditions | Yield |
|---|---|
| With pyridine In water at 70℃; for 0.333333h; | 29% |
-
-
40371-52-6
N-[(S)-4-benzyloxycarbonylamino-2-hydroxy-butyryloxy]succinimide

-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| Stage #1: kanamycin A With zinc diacetate In water; N,N-dimethyl-formamide at 20℃; for 16h; Stage #2: N-[(S)-4-benzyloxycarbonylamino-2-hydroxy-butyryloxy]succinimide In water; N,N-dimethyl-formamide for 24h; | A 12% B 16% |


| Conditions | Yield |
|---|---|
| With pyridine |

| Conditions | Yield |
|---|---|
| at 37℃; aminoglycoside-4'-nucleotidyltransferaze of Bacillus brevis, MgCl2, potassium phosphate buffer (pH 6.0); |
-
-
59-01-8
kanamycin A

| Conditions | Yield |
|---|---|
| With diclazuril In water-d2 |
Kanamycin Chemical Properties
Product Name: Kanamycin (CAS NO.59-01-8)
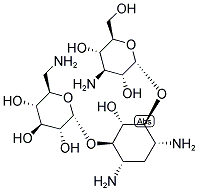
Molecular Formula: C18H36N4O11
Molecular Weight: 484.5g/mol
Mol File: 59-01-8.mol
Einecs: 200-411-7
Boiling point: 809.5 °C at 760 mmHg
Storage Temperature: 2-8°C
Flash Point: 443.4 °C
Density: 1.62 g/cm3
Surface Tension: 104.4 dyne/cm
Enthalpy of Vaporization: 134 kJ/mol
Vapour Pressure: 2.47E-30 mmHg at 25°C
XLogP3-AA: -6.9
H-Bond Donor: 11
H-Bond Acceptor: 15
Kanamycin Uses
Kanamycin (CAS NO.59-01-8) is for the production of sulfuric acid amikacin, kanamycin monosulfate, and pairs of kanamycin sulfate intermediates.
It is used in molecular biology as a selective agent most commonly to isolate bacteria (e.g., E. coli) which have taken up genes (e.g., of plasmids) coupled to a gene coding for kanamycin resistance (primarily Neomycin phosphotransferase II [NPT II/Neo]). Bacteria that have been transformed with a plasmid containing the kanamycin resistance gene are plated on kanamycin (50-100mg/L) containing agar plates or are grown in media containing kanamycin (50-100mg/L). Only the bacteria that have successfully taken up the kanamycin resistance gene become resistant and will grow under these conditions. As a powder kanamycin is white to off-white and is soluble in water (50mg/ml).
At least one such gene, Atwbc19 is native to a plant species, of comparatively large size and its coded protein acts in a manner which decreases the possibility of Horizontal Gene Transfer from the plant to bacteria; it may be incapable of giving resistance to kanamycin to bacteria even if gene transfer occurs.
Kanamycin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | intraperitoneal | 1385mg/kg (1385mg/kg) | Antibiotiki. Vol. 19, Pg. 552, 1974. | |
| human | TDLo | unreported | 843mg/kg/17W- (843mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CHANGE IN ACUITY: EAR SENSE ORGANS AND SPECIAL SENSES: TINNITUS: EAR KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION | American Review of Respiratory Disease. Vol. 82, Pg. 23, 1960. |
| mouse | LD50 | intracrebral | 33750ug/kg (33.75mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 17, Pg. 1664, 1969. |
| mouse | LD50 | intramuscular | 54mg/kg (54mg/kg) | Bollettino Chimico Farmaceutico. Vol. 98, Pg. 224, 1959. | |
| mouse | LD50 | intraperitoneal | 794mg/kg (794mg/kg) | Chemotherapy Vol. 16, Pg. 371, 1971. | |
| mouse | LD50 | intravenous | 115mg/kg (115mg/kg) | Minerva Farmaceutica. Vol. 11, Pg. 108, 1962. | |
| mouse | LD50 | oral | 20700ug/kg (20.7mg/kg) | Antibiotiki. Vol. 19, Pg. 552, 1974. | |
| mouse | LD50 | subcutaneous | 1350mg/kg (1350mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 341, 1967. | |
| rabbit | LD50 | intravenous | 150mg/kg (150mg/kg) | "Antibiotics: Origin, Nature, and Properties," Korzyoski, T., et al., eds., Washington, DC, American Soc. for Microbiology, 1978Vol. 1, Pg. 690, 1978. | |
| rat | LD50 | intramuscular | > 1014mg/kg (1014mg/kg) | BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: CYANOSIS LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Chemotherapy Vol. 29(Suppl, |
| rat | LD50 | intraperitoneal | 1515mg/kg (1515mg/kg) | Antibiotiki. Vol. 19, Pg. 552, 1974. | |
| rat | LD50 | intravenous | 437mg/kg (437mg/kg) | Chemotherapy Vol. 29(Suppl, | |
| rat | LD50 | oral | > 10gm/kg (10000mg/kg) | Chemotherapy Vol. 29(Suppl, | |
| rat | LD50 | subcutaneous | 4070mg/kg (4070mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 176, Pg. 59, 1968. |
Kanamycin Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Kanamycin Safety Profile
Poison by intravenous and intramuscular routes. Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Kanamycin Specification
Kanamycin ,its CAS NO. is 59-01-8,the synonyms is Kanamycin a ; Kanamycin base ; 2-Deoxy-y-alpha-d-glucopyranosyl- ; 4,6-Diamino-2-hydroxy-1,3-cyclohexane3,6'diamino-3,6'-dideoxydi-alpha-d-gluc ; 4,6-Diamino-2-hydroxy-1,3-cyclohexane3,6'diamino-3,6'-dideoxydi-alpha-d-glucos ; D-deoxydi ; D-streptamine,o-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1.fwdarw.6)-o-6-amin .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View