-
Name
Latanoprost
- EINECS 634-172-9
- CAS No. 130209-82-4
- Article Data48
- CAS DataBase
- Density 1.093 g/cm3
- Solubility Soluble in: DMSO (25 mg/mL); ethanol (25 mg/mL)
- Melting Point
- Formula C26H40O5
- Boiling Point 583.8 °C at 760 mmHg
- Molecular Weight 432.601
- Flash Point 188.3 °C
- Transport Information
- Appearance pale yellow oil
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Lata prostaglandin;Latanoprost [USAN:BAN:INN];XA 41;Xalatan (TN);5-Heptenoic acid, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenylpentyl)cyclopentyl)-, 1-methylethyl ester, (1R-(1-alpha(Z),2-beta(R*),3-alpha,5-alpha))-;Xalatan;PhXA 41;Isopropyl (Z)-7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((3R)-3-hydroxy-5-phenylpentyl)cyclopentyl)-5-heptenoate;Latanoprost (JAN/USAN);
- PSA 86.99000
- LogP 4.18640
Synthetic route

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetone at 20℃; for 14h; | A n/a B 99.83% |


| Conditions | Yield |
|---|---|
| With Lipase Novozym 435 at 30℃; for 18h; Enzymatic reaction; | 92% |
| Novozym 435 at 30℃; for 18h; | 92% |
| With 2-chloro-1,3-dimethyl imidazolium chloride; potassium tert-butylate; triethylamine at 0 - 70℃; for 2h; |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In methanol at 52℃; for 4h; Product distribution / selectivity; | 90% |
| pyridinium p-toluenesulfonate In methanol at 52℃; for 4h; | 90% |
| With pyridinium p-toluenesulfonate In dichloromethane at 40℃; Inert atmosphere; | 86.1% |
| With toluene-4-sulfonic acid In isopropyl alcohol at 40℃; for 5h; | |
| With ethanol; pyridinium p-toluenesulfonate at 20 - 50℃; for 3h; |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 84% |
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 14h; | 72% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; | 68% |
-
-
856453-33-3
C41H64O8

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In methanol at 45℃; for 6h; Product distribution / selectivity; | 83.7% |
| pyridinium p-toluenesulfonate In methanol at 45℃; for 6h; | 83.7% |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Stage #1: C39H46O7 With hydrogen In ethyl acetate at 20℃; for 4h; Inert atmosphere; Stage #2: With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 0℃; for 0.5h; Inert atmosphere; Stage #3: With sodium tetrahydroborate In methanol; dichloromethane; water at 0℃; for 2.5h; Inert atmosphere; | 83% |
-
-
141074-06-8
C26H38O5

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Stage #1: C26H38O5 With 2,6-di-tert-butyl-4-methyl-phenol; diisobutylaluminium hydride In toluene at -70 - -20℃; Stage #2: With hydrogenchloride In water; toluene | 75% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetone at 20℃; for 5.5 - 14.2167h; Product distribution / selectivity; | A n/a B n/a C 63.2% |


| Conditions | Yield |
|---|---|
| Stage #1: C23H32O4 With sodium hydride In isopropyl alcohol; mineral oil at 20 - 35℃; for 18h; Stage #2: 2-iodo-propane With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; for 18h; | 49% |
-
-
477884-73-4
(3aR,4R,5R,6aS)-hexahydro-5-triethylsilyoxy-4-[5-phenyl-(3R)-3-triethylsilyloxy-pentanyl]-2H-cyclopenta[β]furan-2-one



-
C
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Purification / work up; | A n/a B n/a C 45% |

-
-
31752-99-5
(-)-Corey lactone 5-(4-phenylbenzoate)

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: DCC, phosphoric acid / 1,2-dimethoxy-ethane / a) 18 deg C, 30 min, b) RT, 2 h 2: 1.) NaH / 1.) DME, RT, 1 h, 2.) DME, a) 0 deg C, 30 min, b) RT, 2 h 3: 52 percent / lithium tri-sec-butylborohydride (lithium selectride) / tetrahydrofuran; diethyl ether / 1 h / -78 - -75 °C 4: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 5: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 6: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 7: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 8: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 1.) NaH, 2.) n-BuLi 2: 1.) NaH / 1.) DME, RT, 1 h, 2.) DME, a) 0 deg C, 30 min, b) RT, 2 h 3: 52 percent / lithium tri-sec-butylborohydride (lithium selectride) / tetrahydrofuran; diethyl ether / 1 h / -78 - -75 °C 4: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 5: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 6: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 7: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 8: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
41639-72-9
(1S,5R,6R,7R)-6-(3-oxo-5-phenyl-1E-pentenyl)-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 52 percent / lithium tri-sec-butylborohydride (lithium selectride) / tetrahydrofuran; diethyl ether / 1 h / -78 - -75 °C 2: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 3: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 4: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 5: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 6: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
41639-23-0
(1S,5R,6R,7R)-6-<(3S)-3-hydroxy-5-phenyl-1-pentenyl>-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 2: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 3: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 4: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 5: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 1.) NaH / 1.) DME, RT, 1 h, 2.) DME, a) 0 deg C, 30 min, b) RT, 2 h 2: 52 percent / lithium tri-sec-butylborohydride (lithium selectride) / tetrahydrofuran; diethyl ether / 1 h / -78 - -75 °C 3: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 4: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 5: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 6: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 7: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 2: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
145773-21-3
(1S,5R,6R,7R)-6-<(3R)-3-hydroxy-5-phenyl-1-pentyl>-7(R)-hydroxy-2-oxabicyclo<3.3.0>octan-3-one

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 2: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 3: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
145773-20-2
(1S,5R,6R,7R)-6-<(3R)-3-hydroxy-5-phenyl-1-pentyl>-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 2: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 3: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 4: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
38754-71-1
(3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 1.) NaH / 1.) DME, RT, 1 h, 2.) DME, a) 0 deg C, 30 min, b) RT, 2 h 2: 52 percent / lithium tri-sec-butylborohydride (lithium selectride) / tetrahydrofuran; diethyl ether / 1 h / -78 - -75 °C 3: 78.4 percent / H2, 1 M NaOH / 10percent Pd/C / ethanol / 6 h 4: 85 percent / K2CO3 / methanol / 6 h / Ambient temperature 5: 65 percent / diisobutylaluminium hydride (DIBAL) / toluene; tetrahydrofuran / 1 h / -80 - -72 °C 6: 1.) t-BuOK / 1.) THF, RT, 30 min, 2.) THF, -15 to -10 deg C, 4 h 7: 38 percent / DBU / acetone / 4 h / Ambient temperature View Scheme |
-
-
41639-83-2
latanoprost acid

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With caesium carbonate In water; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In acetone at 0 - 25℃; pH=1 - 3; |
-
-
477884-76-7
C38H68O5Si2

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; for 0.5h; |


| Conditions | Yield |
|---|---|
| In methanol; water | |
| In methanol; water |
-
-
474944-22-4
(Z)-isopropyl 7-((1R,2R,3R,5S)-3-(tert-butyldimethylsilyloxy)-2-((R)-3-(tert-butyldimethylsilyloxy)-5-phenylpentyl)-5-hydroxycyclopentyl)hept-5-enoate

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In isopropyl alcohol at 25 - 30℃; |

| Conditions | Yield |
|---|---|
| Stage #1: latanoprost acid With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetone at 20 - 25℃; for 0.166667h; Stage #2: isopropyl bromide In acetone for 0.5h; |
-
-
876953-73-0
tert-butyl[(1S)-3-iodo-1-phenethylpropyl]oxydimethylsilane

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: tert.-butyl lithium / hexane; diethyl ether / 4 h / -78 °C / Inert atmosphere 1.2: 0.92 h / -78 - -50 °C / Inert atmosphere 2.1: diisobutylaluminium hydride / toluene / 3 h / -78 °C 2.2: -50 °C 3.1: dmap; diisopropyl-carbodiimide / tetrahydrofuran / 24 h / 20 - 40 °C 4.1: Grubbs catalyst first generation / dichloromethane / 18 h / 20 - 40 °C / Inert atmosphere 5.1: tetrabutyl ammonium fluoride; ammonium hydrogen difluoride / tetrahydrofuran / 24 h / 20 - 40 °C 6.1: sodium hydride / isopropyl alcohol; mineral oil / 18 h / 20 - 35 °C 6.2: 18 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: tert.-butyl lithium / diethyl ether; pentane / 4 h / -78 - -40 °C / Inert atmosphere; Schlenk technique 1.2: 0.5 h / -30 °C / Inert atmosphere; Schlenk technique 2.1: ozone / methanol; dichloromethane / -78 °C 2.2: -78 - 20 °C 3.1: hydrogenchloride; water / tetrahydrofuran / 16 h / 20 °C 4.1: potassium tert-butylate / tetrahydrofuran / 0.67 h / 0 °C / Inert atmosphere; Schlenk technique 4.2: 0 - 20 °C / Inert atmosphere; Schlenk technique 5.1: caesium carbonate / N,N-dimethyl-formamide / 18 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: tert.-butyl lithium / diethyl ether; pentane / 4 h / -78 - -40 °C / Inert atmosphere; Schlenk technique 1.2: 0.5 h / -30 °C / Inert atmosphere; Schlenk technique 2.1: ozone / methanol; dichloromethane / -78 °C 2.2: -78 - 20 °C 3.1: diisobutylaluminium hydride / dichloromethane / 2 h / -78 °C 4.1: valerianate de potassium / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere; Schlenk technique 4.2: 0 - 20 °C / Inert atmosphere; Schlenk technique 5.1: hydrogenchloride; water / tetrahydrofuran / 17 h / 20 °C 6.1: caesium carbonate / N,N-dimethyl-formamide / 18 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: tert.-butyl lithium / diethyl ether / 4 h / -78 - -40 °C / Inert atmosphere 1.2: 1 h / -78 - -20 °C / Inert atmosphere 2.1: ozone / methanol; dichloromethane / -78 °C 2.2: 3.25 h / -78 - 20 °C / Inert atmosphere 3.1: hydrogenchloride; water / tetrahydrofuran / 16 h / 20 °C 4.1: potassium tert-butylate / tetrahydrofuran / 0.67 h / 0 °C / Inert atmosphere 4.2: 1.5 h / 0 - 20 °C / Inert atmosphere 5.1: caesium carbonate / N,N-dimethyl-formamide / 18 h / 20 °C View Scheme |
-
-
1261926-67-3
C31H54O3Si2

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: diisobutylaluminium hydride / toluene / 3 h / -78 °C 1.2: -50 °C 2.1: dmap; diisopropyl-carbodiimide / tetrahydrofuran / 24 h / 20 - 40 °C 3.1: Grubbs catalyst first generation / dichloromethane / 18 h / 20 - 40 °C / Inert atmosphere 4.1: tetrabutyl ammonium fluoride; ammonium hydrogen difluoride / tetrahydrofuran / 24 h / 20 - 40 °C 5.1: sodium hydride / isopropyl alcohol; mineral oil / 18 h / 20 - 35 °C 5.2: 18 h / 20 °C View Scheme |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 16h; | 94% |
-
-
130209-82-4
latanoprost

-
-
41639-83-2
latanoprost acid

| Conditions | Yield |
|---|---|
| Stage #1: latanoprost With lithium hydroxide; water In tetrahydrofuran for 16h; Stage #2: With hydrogenchloride In tetrahydrofuran; water | 90% |
-
-
4426-47-5
butylboronic acid

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| In dichloromethane at 45℃; for 20h; Inert atmosphere; | 80% |
| In dichloromethane at 45℃; for 22h; Inert atmosphere; |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 28h; Inert atmosphere; | 60% |

-
-
130209-82-4
latanoprost

-
-
propan-2-yl (5Z)-7-[3,5-bis({[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-(acetyloxy)propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy})-2-(3-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-(acetyloxy)propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}-5-phenylpentyl)cyclopentyl]hept-5-enoate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 48h; Inert atmosphere; | 54% |

-
-
130209-82-4
latanoprost

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 48h; Inert atmosphere; | 37% |

-
-
130209-82-4
latanoprost

-
-
propan-2-yl (5Z)-7-[3,5-bis({[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-(acetyloxy)propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy})-2-(3-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-(acetyloxy)propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}propanoyl]oxy}-5-phenylpentyl)cyclopentyl]hept-5-enoate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 30℃; for 48h; Inert atmosphere; | 35% |
Latanoprost Chemical Properties
Formula: C26H40O5
Mol. mass :432.593 g/mol
Other name:Latanoprost
XALATAN the brand name of Xalatan manufactured by Pfizer.It is freely soluble in DMSO.
Following is the molecular structure of XALATAN: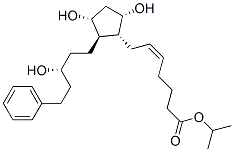
Latanoprost Uses
XALATAN is also a prostaglandin analogue that works by increasing the outflow of aqueous fluid from the eyes.
Latanoprost Toxicity Data With Reference
| 1. | ocu-man TDLo:33 µL/kg/25D-I:EYE | AJOPAA American Journal of Ophthalmology. 124 (1997),683. | ||
| 2. | ocu-man TDLo:99 µL/kg/20W-I:SKN | AJOPAA American Journal of Ophthalmology. 124 (1997),544. | ||
| 3. | ocu-wmn TDLo:4 nL/kg/5W-I:EYE | AJOPAA American Journal of Ophthalmology. 127 (1999),91. | ||
| 4. | orl-rat LD50:>50 µg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 42 (2000),195. | ||
| 5. | ivn-rat LD50:>2 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 42 (2000),195. | ||
| 6. | orl-mus LD50:>50 µg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 42 (2000),195. | ||
| 7. | ivn-mus LD50:>2 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 42 (2000),195. | ||
| 8. | ivn-dog LD50:>680 µg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 42 (2000),195. |
RTECS : MJ9669550
Latanoprost Safety Profile
Related Products
- Latanoprost
- Latanoprost acid
- Latanoprost Lactol
- Latanoprost Lactone Diol
- 13021-14-2
- 13021-15-3
- 13021-18-6
- 13021-40-4
- 130216-67-0
- 130216-69-2
- 130217-20-8
- 130217-31-1
- 130221-78-2
- 130-22-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View