-
Name
Lopinavir
- EINECS
- CAS No. 192725-17-0
- Article Data20
- CAS DataBase
- Density 1.163 g/cm3
- Solubility
- Melting Point 124-127 °C
- Formula C37H48N4O5
- Boiling Point 924.1 °C at 760 mmHg
- Molecular Weight 628.812
- Flash Point 512.7 °C
- Transport Information
- Appearance white crystalline solid
- Safety
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1(2H)-Pyrimidineacetamide,N-[(1S,3S,4S)-4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2-oxo-, (aS)- (9CI);1(2H)-Pyrimidineacetamide,N-[4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2-oxo-, [1S-[1R*(R*),3R*,4R*]]-;A157378.0;ABT 378;Aluviran;Koletra;1(2H)-Pyrimidineacetamide,N-[(1S,3S,4S)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2-oxo-, (aS)-;Intermediates of Lopinavir;
- PSA 120.00000
- LogP 4.39310
Synthetic route

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| In ethyl acetate at 25℃; for 8h; Solvent; | 97% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 8h; Solvent; | 96% |
-
-
224648-11-7
N-(2,6-dimethyl-phenyloxy acetyloxy)succinimide

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 96% |
-
-
192800-77-4
2S-(1-tetrahydro-pyrimid-2-onyl)-3-methyl butanoyl chloride

-
-
192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With 1H-imidazole In ethyl acetate; N,N-dimethyl-formamide at 0 - 20℃; Reagent/catalyst; Inert atmosphere; | 92% |
| With 1H-imidazole; dmap In ethyl acetate; N,N-dimethyl-formamide at 0 - 10℃; for 14h; |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 92% |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 92% |
-
-
192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-50-1
(S)-tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetic acid

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane; (S)-tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetic acid With thionyl chloride In dichloromethane at 0℃; for 2h; Reflux; Stage #2: With triethylamine In dichloromethane at 0 - 20℃; for 5h; | 90.1% |
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide | 70% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 25℃; for 8h; Solvent; | 89% |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 89% |
-
-
192725-84-1
(S)-2-(3-Amino-propylamino)-3-methyl-butyric acid methyl ester

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: LiOH 2: EDAC; HOBt View Scheme |
-
-
192725-85-2
(S)-3-Methyl-2-(2-oxo-tetrahydro-pyrimidin-1-yl)-butyric acid methyl ester

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LiOH 2: EDAC; HOBt View Scheme |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: trifluoroacetic acid 2: LiOH 3: EDAC; HOBt View Scheme |
-
-
144163-85-9
(2S,3S,5S)-2-amino-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDC 2: trifluoroacetic acid View Scheme | |
| Multi-step reaction with 3 steps 1.1: thionyl chloride; N,N-dimethyl-formamide / ethyl acetate / 4 h / 50 °C 1.2: 5.25 h / 20 °C 2.1: trifluoroacetic acid / dichloromethane / 0 h / 0 - 25 °C / Inert atmosphere 3.1: triethylamine; benzotriazol-1-ol / N,N-dimethyl-formamide / 0.33 h / 0 °C / Inert atmosphere 3.2: 12 h / 20 - 25 °C / Inert atmosphere View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Pd(OH)2; HCO2NH4 2: EDC 3: trifluoroacetic acid View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1,1'-carbonyldiimidazole / ethyl acetate / 2 h / 15 - 20 °C 1.2: 4 h / 70 - 75 °C 2.1: 5%-palladium/activated carbon; ammonium formate / methanol / 2 h / 50 - 55 °C 3.1: sodium hydrogencarbonate / water; ethyl acetate / 0.5 h / 10 - 15 °C View Scheme |
-
-
162849-93-6
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Pd(OH)2; HCO2NH4 2: EDC 3: trifluoroacetic acid View Scheme |
-
-
192725-45-4
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic acid View Scheme |
-
-
192726-05-9
(2S,3S,5S)-2-amino-3-hydroxy-5-(2S-(2-oxotetrahydropyrimidin-2-yl)-3-methylbutanoyl)amino-1,6-diphenylhexane

-
-
20143-48-0
2,6-dimethylphenoxyacetyl chloride

-
-
192725-17-0
lopinavir
-
-
192800-77-4
2S-(1-tetrahydro-pyrimid-2-onyl)-3-methyl butanoyl chloride

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane With 1H-imidazole In ethyl acetate at 20℃; Stage #2: 2S-(1-tetrahydro-pyrimid-2-onyl)-3-methyl butanoyl chloride In ethyl acetate; N,N-dimethyl-formamide at 0 - 20℃; for 13h; | |
| Multi-step reaction with 4 steps 1: 1H-imidazole / ethyl acetate; N,N-dimethyl-formamide / 2 - 30 °C / Inert atmosphere 2: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 3: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 4: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme |
-
-
192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane With 1H-imidazole In ethyl acetate; N,N-dimethyl-formamide at 0 - 25℃; for 13h; Stage #2: With hydrogenchloride In water; ethyl acetate; N,N-dimethyl-formamide at 10 - 15℃; |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 20 - 25℃; for 1.16667h; |
-
-
192725-50-1
(S)-tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetic acid

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: thionyl chloride / tetrahydrofuran / 5.5 h / 4 - 20 °C / Inert atmosphere 2: 1H-imidazole / ethyl acetate; N,N-dimethyl-formamide / 2 - 30 °C / Inert atmosphere 3: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 4: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 5: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1,1'-carbonyldiimidazole / ethyl acetate / 2 h / 15 - 20 °C 1.2: 4 h / 70 - 75 °C 2.1: 5%-palladium/activated carbon; ammonium formate / methanol / 2 h / 50 - 55 °C 3.1: sodium hydrogencarbonate / water; ethyl acetate / 0.5 h / 10 - 15 °C View Scheme |
-
-
72-18-4
L-valine

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: lithium chloride; lithium hydroxide; aluminum oxide / water / 5 h / -20 - -10 °C / pH 9.5 - 10.2 / Inert atmosphere 2: sodium hydroxide / tetrahydrofuran / 2 h / 2 - 10 °C / Inert atmosphere 3: potassium tert-butylate / tetrahydrofuran / 18 h / 20 °C / Inert atmosphere 4: thionyl chloride / tetrahydrofuran / 5.5 h / 4 - 20 °C / Inert atmosphere 5: 1H-imidazole / ethyl acetate; N,N-dimethyl-formamide / 2 - 30 °C / Inert atmosphere 6: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 7: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 8: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: potassium hydroxide / water / 2 h / 0 - 5 °C 2.1: sodium hydroxide / water / pH 9.5 - 10.5 3.1: ammonia; hydrogen / methanol / 10 h / 50 °C 4.1: sodium hydroxide / water / 98 - 100 °C 5.1: 1,1'-carbonyldiimidazole / ethyl acetate / 2 h / 15 - 20 °C 5.2: 4 h / 70 - 75 °C 6.1: 5%-palladium/activated carbon; ammonium formate / methanol / 2 h / 50 - 55 °C 7.1: sodium hydrogencarbonate / water; ethyl acetate / 0.5 h / 10 - 15 °C View Scheme |
-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: sodium hydroxide / tetrahydrofuran / 2 h / 2 - 10 °C / Inert atmosphere 2: potassium tert-butylate / tetrahydrofuran / 18 h / 20 °C / Inert atmosphere 3: thionyl chloride / tetrahydrofuran / 5.5 h / 4 - 20 °C / Inert atmosphere 4: 1H-imidazole / ethyl acetate; N,N-dimethyl-formamide / 2 - 30 °C / Inert atmosphere 5: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 6: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 7: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: potassium tert-butylate / tetrahydrofuran / 18 h / 20 °C / Inert atmosphere 2: thionyl chloride / tetrahydrofuran / 5.5 h / 4 - 20 °C / Inert atmosphere 3: 1H-imidazole / ethyl acetate; N,N-dimethyl-formamide / 2 - 30 °C / Inert atmosphere 4: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 5: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 6: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 5%-palladium/activated carbon; ammonium formate / methanol / 50 °C / Inert atmosphere 2: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 3: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1,4-dioxane / 1 h / 20 - 50 °C / Inert atmosphere 2: sodium hydrogencarbonate / ethyl acetate; water / 0.58 h / 20 °C / Inert atmosphere View Scheme |

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydrogencarbonate / tert-butyl methyl ether; water / 1 h / 25 - 35 °C 1.2: 45 - 60 °C 2.1: ammonium formate; 5%-palladium/activated carbon / methanol / 50 - 55 °C 2.2: 1 h / 50 - 55 °C 3.1: sodium hydrogencarbonate / water; ethyl acetate / 1 h / 25 - 35 °C View Scheme |
-
-
67746-43-4
dibenzyl N,N-diethylphosphoramidite

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Stage #1: dibenzyl N,N-diethylphosphoramidite; lopinavir With 1H-tetrazole In tetrahydrofuran at 20℃; for 68h; Stage #2: With 3-chloro-benzenecarboperoxoic acid In tetrahydrofuran; dichloromethane at -45℃; for 0.5h; | 90% |
-
-
170436-73-4
4-{[bis(benzyloxy)phosphoryl]oxy}-3,3-dimethylbutanoic acid

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 19h; | 84% |
-
-
67-68-5
dimethyl sulfoxide

-
-
192725-17-0
lopinavir

-
-
875435-49-7
(2S)-N-{(1S,3S,4S)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-[(methylthio)methoxy]-5-phenylpentyl}-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide

| Conditions | Yield |
|---|---|
| With acetic anhydride at 20℃; for 48h; | 64% |
| With acetic anhydride; acetic acid at 20℃; for 48 - 72h; Product distribution / selectivity; | 64% |
-
-
352-93-2
diethyl sulphide

-
-
192725-17-0
lopinavir

-
-
875435-61-3
(2S)-N-{(1S,3S,4S)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-[1-(ethylthio)ethoxy]-5-phenylpentyl}-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 61% |
| With dibenzoyl peroxide In acetonitrile at 0℃; for 3h; Product distribution / selectivity; | 61% |
| Stage #1: diethyl sulphide With N-chloro-succinimide In tetrahydrofuran at -10℃; Stage #2: lopinavir With triethylamine In tetrahydrofuran at -10℃; for 1.5h; Product distribution / selectivity; |
-
-
875435-99-7
dibenzyl 4-chloro-3,3-dimethyl-4-oxobutyl phosphate

-
-
192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; for 16h; | 56% |

| Conditions | Yield |
|---|---|
| With 1H-tetrazole In tetrahydrofuran at 20℃; for 68h; |
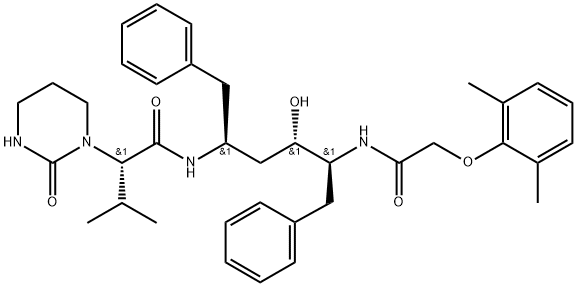
Lopinavir Chemical Properties
IUPAC Name: (2S)-N-[(2S,4S,5S)-5-[[2-(2,6-Dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
Formula: C37H48N4O5
Molecular Weight: 628.8 g/mol
Molecular Structure of Lopinavir (CAS NO.192725-17-0):
Melting Point: 124-127 °C
Flash Point: 512.7 °C
Boiling Point: 924.1 °C at 760 mmHg
Density: 1.163 g/cm3
Enthalpy of Vaporization: 140.81 kJ/mol
Appearance: white crystalline solid
Index of Refraction: 1.577
Molar Refractivity: 179.17 cm3
Molar Volume: 540.4 cm3
Surface Tension: 49.4 dyne/cm
XLogP3-AA: 5.9
H-Bond Donor: 4
H-Bond Acceptor: 5
Rotatable Bond Count: 15
Tautomer Count: 8
Exact Mass: 628.362471
MonoIsotopic Mass: 628.362471
Topological Polar Surface Area: 120
Heavy Atom Count: 46
Product Categories: Anti-viral Compounds;Anti-virals;Intermediates & Fine Chemicals;Non-nucleoside Reverse Transcriptase;Pharmaceuticals
Lopinavir History
In an attempt to improve on the HIV resistance and serum protein-binding properties of the company's earlier protease inhibitor, ritonavir, Lopinavir was developed by Abbott. Abbott pursued a strategy of co-administering lopinavir with sub-therapeutic doses of ritonavir, and lopinavir is only marketed as a co-formulation with ritonavir. It is the first multi-drug capsule to contain a drug not available individually. Lopinavir/ritonavir was approved by the US FDA on 15 September 2000, and in Europe in April 2001. On June 26, 2016, Its patent will expire in the US. Abbott Laboratories was one of the earliest users of the Advanced Photon Source, a national synchrotron-radiation light source at Argonne National Laboratory. One of the early research projects undertaken at the Advanced Photon Source was the Human Immunodeficiency Virus. Using X-ray crystallography, researchers found the points of attack of the HIV protease inhibitors–agents that block the breakdown of proteins. Protease inhibitors stop HIV from making new copies of itself by blocking the last step in the process, when the virus attempts to replicate - and out of that discovery came the drug Kaletra.
Lopinavir Uses
Lopinavir (CAS NO.192725-17-0) is used as a selective HIV protease inhibitor. It is also used as an analogue of Ritonavir.
Lopinavir Specification
The chemical synonyms of Lopinavir (CAS NO.192725-17-0) is also named as (1S-(1R*(R*),3R*,4R*))-N-(4-(((2,6-Dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide ; (alphaS)-Tetrahydro-N-((alphaS)-alpha-((2S,3S)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-alpha-isopropyl-2-oxo-1(2H)-pyrimidineacetamide ; A 157378.0 ; ABT 378 ; ABT-378 ; UNII-2494G1JF75 .
Related Products
- Lopinavir
- 192725-45-4
- 192725-49-8
- 192725-50-1
- 192725-55-6
- 1927-25-9
- 192726-05-9
- 192726-06-0
- 1927-31-7
- 19275-44-6
- 19275-46-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View