-
Name
lupanine
- EINECS
- CAS No. 550-90-3
- Article Data18
- CAS DataBase
- Density 1.16g/cm3
- Solubility
- Melting Point 40-44°
- Formula C15H24 N2 O
- Boiling Point 396.7°Cat760mmHg
- Molecular Weight 248.368
- Flash Point 172.7°C
- Transport Information
- Appearance
- Safety Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 7,14-Methano-2H,11H-dipyrido[1,2-a:1',2'-e][1,5]diazocin-11-one,dodecahydro-, [7S-(7a,7aa,14a,14ab)]-; Lupanine (8CI); Lupanine, d- (6CI); (+)-2-Oxosparteine; (+)-Lupanine;2-Oxosparteine; 7,14-Methano-4H,6H-dipyrido[1,2-a:1',2'-e][1,5]diazocin-4-one,dodecahydro-, [7S-(7a,7ab,14a,14aa)]-; Lupanin; d-Lupanine
- PSA 23.55000
- LogP 1.74750
Synthetic route

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; for 2.5h; | 93% |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water | 30% |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water | 19% |
-
-
3019-47-4, 74183-21-4, 74183-22-5, 133163-04-9
lupanine N(16)-oxide

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; formic acid; sulfur dioxide Ambient temperature; other N-oxides of sparteine lactams; | |
| With hydrogen; platinum(IV) oxide In acetic acid under 760 Torr; for 1h; Product distribution; other N-oxides of sparteine lactams; other times; |
-
-
3019-47-4, 74183-21-4, 74183-22-5, 133163-04-9
lupanine N(16)-oxide

-
B
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With formic acid; sulfur dioxide for 0.5h; Mechanism; Product distribution; Ambient temperature; other N-oxides of sparteine lactams; other times; |
-
-
32101-29-4
5,6-dehydrolupanine

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | 3.2 mg |
| With hydrogen; palladium on activated charcoal In ethanol Ambient temperature; |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With sulfur dioxide In methanol at 0℃; for 0.25h; | 4 mg |


| Conditions | Yield |
|---|---|
| at 170℃; folgende Hydrierung; |


-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CHCl3 / 12 h / Ambient temperature 2: 3.2 mg / H2 / 5percent Pd-C / ethanol View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; for 2.5h; | 93% |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water | 30% |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water | 19% |
-
-
3019-47-4, 74183-21-4, 74183-22-5, 133163-04-9
lupanine N(16)-oxide

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; formic acid; sulfur dioxide Ambient temperature; other N-oxides of sparteine lactams; | |
| With hydrogen; platinum(IV) oxide In acetic acid under 760 Torr; for 1h; Product distribution; other N-oxides of sparteine lactams; other times; |
-
-
3019-47-4, 74183-21-4, 74183-22-5, 133163-04-9
lupanine N(16)-oxide

-
B
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With formic acid; sulfur dioxide for 0.5h; Mechanism; Product distribution; Ambient temperature; other N-oxides of sparteine lactams; other times; |
-
-
32101-29-4
5,6-dehydrolupanine

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | 3.2 mg |
| With hydrogen; palladium on activated charcoal In ethanol Ambient temperature; |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With sulfur dioxide In methanol at 0℃; for 0.25h; | 4 mg |


| Conditions | Yield |
|---|---|
| at 170℃; folgende Hydrierung; |


-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CHCl3 / 12 h / Ambient temperature 2: 3.2 mg / H2 / 5percent Pd-C / ethanol View Scheme |
-
-
17026-42-5
O,O'-dibenzoyl-D-tartaric acid

-
-
4356-43-8
rac-lupanine

-
A
-
1335287-92-7
(-)-lupanine (+)-2,3-dibenzoyl-D-tartarate

-
B
-
550-90-3
(+)-lupanine

-
C
-
486-88-4
L-(-)-lupanine

| Conditions | Yield |
|---|---|
| In methanol Resolution of racemate; Heating; optical yield given as %ee; |


| Conditions | Yield |
|---|---|
| Stage #1: L-Tartaric acid; rac-lupanine In acetone Resolution of racemate; Stage #2: With potassium hydroxide In water Resolution of racemate; | n/a |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: triethylamine; dmap / dichloromethane / 20 h 2.1: triethylamine / tetrahydrofuran / 5 h / 20 °C 3.1: Adam’s catalyst; hydrogen / methanol / 4 h / 20 °C / 760.05 Torr 4.1: sodium tetrahydroborate / 2.5 h / 0 °C 4.2: 4 h / 0 - 20 °C 5.1: boron trifluoride diethyl etherate / dichloromethane / 17 h / 0 - 20 °C 6.1: triethylamine / dichloromethane / 120 h 7.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 8.1: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: triethylamine / tetrahydrofuran / 5 h / 20 °C 2.1: Adam’s catalyst; hydrogen / methanol / 4 h / 20 °C / 760.05 Torr 3.1: sodium tetrahydroborate / 2.5 h / 0 °C 3.2: 4 h / 0 - 20 °C 4.1: boron trifluoride diethyl etherate / dichloromethane / 17 h / 0 - 20 °C 5.1: triethylamine / dichloromethane / 120 h 6.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 7.1: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: Adam’s catalyst; hydrogen / methanol / 4 h / 20 °C / 760.05 Torr 2.1: sodium tetrahydroborate / 2.5 h / 0 °C 2.2: 4 h / 0 - 20 °C 3.1: boron trifluoride diethyl etherate / dichloromethane / 17 h / 0 - 20 °C 4.1: triethylamine / dichloromethane / 120 h 5.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 6.1: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium tetrahydroborate / 2.5 h / 0 °C 1.2: 4 h / 0 - 20 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 17 h / 0 - 20 °C 3.1: triethylamine / dichloromethane / 120 h 4.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 5.1: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: boron trifluoride diethyl etherate / dichloromethane / 17 h / 0 - 20 °C 2: triethylamine / dichloromethane / 120 h 3: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 4: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 120 h 2: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 3: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |

-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 1 h / Reflux 2: palladium 10% on activated carbon; hydrogen / methanol / 2.5 h / 20 °C / 760.05 Torr View Scheme |
-
-
550-90-3
(+)-lupanine

-
-
3019-47-4, 74183-21-4, 74183-22-5, 133163-04-9
lupanine N(16)-oxide

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 48h; | 90% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran Heating; | 85% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 18h; Reflux; | 84% |
| With hydrogenchloride; platinum Hydrogenation; | |
| With lithium aluminium tetrahydride In diethyl ether for 5h; Heating; |
-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane | 80% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 6h; Ambient temperature; | 71% |
-
-
506-68-3
bromocyane

-
-
550-90-3
(+)-lupanine

-
-
2584-20-5
(1S)-4c-(4-bromo-butyl)-8-oxo-(11ac)-octahydro-1r,5c-methano-pyrido[1,2-a][1,5]diazocine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With benzene |
-
-
550-90-3
(+)-lupanine

-
-
74-88-4
methyl iodide

-
-
6080-95-1, 54815-53-1, 58846-15-4, 82978-45-8, 82978-46-9, 115113-81-0
N-16-methyl-2-oxosparteine iodide

| Conditions | Yield |
|---|---|
| at 110 - 120℃; im Rohr; | |
| for 96h; Ambient temperature; Yield given; |
-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide; potassium polysulfide; xylene |
-
-
550-90-3
(+)-lupanine

-
-
15043-17-1, 55732-48-4, 69427-33-4, 127515-92-8
17-hydroxylupanine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; chloroform und anschliessenden Behandeln mit wss.NaOH; | |
| With methanol; silver(l) oxide | |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,4-dioxane |
-
-
550-90-3
(+)-lupanine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride anschliessend Behandeln mit Eis; |

| Conditions | Yield |
|---|---|
| With potassium permanganate; acetic acid; acetone | |
| With sodium hydroxide; potassium hexacyanoferrate(III) |
-
-
550-90-3
(+)-lupanine

-
-
15333-74-1, 33964-84-0, 117895-46-2
4-((1S)-(11at)-decahydro-1r,5c-methano-pyrido[1,2-a][1,5]diazocin-4t-yl)-butyric acid ; dihydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| In acetone for 72h; |
-
-
550-90-3
(+)-lupanine

-
-
4697-83-0, 38485-03-9
(+)-2,17-dioxosparteine

| Conditions | Yield |
|---|---|
| With potassium permanganate |
-
-
550-90-3
(+)-lupanine

-
-
19879-75-5, 20210-08-6, 20210-10-0
(2,2-2H2)sparteine

| Conditions | Yield |
|---|---|
| With lithium aluminium deuteride In tetrahydrofuran for 1h; Heating; | 50 mg |
Lupanine Chemical Properties
Product Name: Lupanine
Synonyms: (+)-2-Oxosparteine ; (7S,7aR,14S,14aS)-Dodecahydro-7,14-methano-2H,11H-dipyrido[1,2-a:1',2'-e][1,5]diazocin-11-one ; Oxosparteine ;(+)-Lupanine ; 2-Oxosparteine
CAS NO: 550-90-3
Molecular Formula of Lupanine (CAS NO.550-90-3) : C15H24N2O
Molecular Weight of Lupanine (CAS NO.550-90-3) : 248.3639
Molecular Structure of Lupanine (CAS NO.550-90-3) :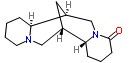
Index of Refraction: 1.581
Surface Tension: 48.3 dyne/cm
Density: 1.16 g/cm3
Flash Point: 172.7 °C
Enthalpy of Vaporization: 64.71 kJ/mol
Boiling Point: 396.7 °C at 760 mmHg
Vapour Pressure: 1.67E-06 mmHg at 25°C
Lupanine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LDLo | intraperitoneal | 22mg/kg (22mg/kg) | Journal of Agricultural Research Vol. 32, Pg. 51, 1926. | |
| guinea pig | LDLo | intravenous | 78mg/kg (78mg/kg) | BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Planta Medica. Vol. 50, Pg. 420, 1984. |
| mouse | LD50 | intraperitoneal | 175mg/kg (175mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Planta Medica. Vol. 50, Pg. 420, 1984. |
| mouse | LD50 | oral | 410mg/kg (410mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Planta Medica. Vol. 50, Pg. 420, 1984. |
| rat | LD50 | intraperitoneal | 177mg/kg (177mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS | JAT, Journal of Applied Toxicology. Vol. 7, Pg. 51, 1987. |
| rat | LD50 | oral | 1440mg/kg (1440mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 210, Pg. 27, 1974. |
Lupanine Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View