-
Name
Lupeol
- EINECS 208-889-9
- CAS No. 545-47-1
- Article Data30
- CAS DataBase
- Density 0.978 g/cm3
- Solubility
- Melting Point 215-216oC
- Formula C30H50O
- Boiling Point 488.1 °C at 760 mmHg
- Molecular Weight 426.726
- Flash Point 216.9 °C
- Transport Information
- Appearance white powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Lup-20(29)-en-3b-ol (8CI);Monogynol B (6CI);(+)-Lupeol;3b-Hydroxylup-20(29)-ene;Clerodol;Fagarasterol;Fagarsterol;Lupenol;NSC 90487;b-Viscol;
- PSA 20.23000
- LogP 8.02480
Synthetic route
-
-
1617-68-1, 6610-54-4, 55870-40-1, 74345-30-5, 101627-42-3
lupeol acetate

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 0.0166667h; microwave irradiation; | 90% |
| With potassium hydroxide In ethanol for 5h; Heating; | 85% |
| With potassium hydroxide In ethanol for 5h; Reflux; | 85% |
-
-
1190597-45-5
tert-butyl((1R,3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a,5a,5b,8,8,11a-hexamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysene-9-yloxy)dimethylsilane

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 50℃; for 24h; Inert atmosphere; | 90% |
-
-
27570-21-4
3-O-acetylbetulinal

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Stage #1: 3-O-acetylbetulinal With hydrazine hydrate In diethylene glycol dimethyl ether at 80℃; for 3h; Stage #2: With potassium hydroxide In diethylene glycol dimethyl ether for 5h; Reagent/catalyst; Temperature; Reflux; | 68% |
| With hydrazine hydrate; potassium hydroxide In diethylene glycol for 6h; Reflux; | 65% |
| Multi-step reaction with 2 steps 1.1: p-toluenesulfonylhydrazine; Na2SO4 / ethanol / 3.5 h / 100 °C 1.2: 14 percent / NaBH3CN; p-toluenesulfonic acid monohydrate / dimethylformamide; tetrahydrothiophene 1,1-dioxide / 1 h / 100 °C 2.1: 70 percent / K2CO3 / methanol / 13 h / 50 °C View Scheme |
-
-
199665-79-7
lupeol-3-(3'R-hydroxy)-hexadecanoate

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water for 38h; Reflux; | 64% |
-
-
13159-28-9, 92594-07-5
betulinic aldehyde

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Stage #1: betulinic aldehyde With hydrazine hydrate In dimethyl sulfoxide at 80℃; for 3h; Stage #2: With potassium tert-butylate In dimethyl sulfoxide at 160℃; for 5h; | 61% |

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 160℃; for 5h; Reagent/catalyst; Temperature; Solvent; | 55% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethylene glycol for 2h; Heating; | A 50% B 15% |
| With aluminum isopropoxide; isopropyl alcohol |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethylene glycol at 160℃; Heating; reflux 2 h, cooled, dil. HCl, ether extr., washed: water, dried, solvent distillation, chromatographed, petrol and benzene:petrol (2:3) elution; Further byproducts given. Yields of byproduct given; | 50% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethylene glycol for 2h; Heating; | A 40% B 8% C 15% |

-
-
1617-70-5
lupenone

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
-
71-43-2
benzene

-
-
545-47-1
lupenol



-
B
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
L-arabinose

-
C
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With sulfuric acid for 5h; Heating; | A n/a B n/a C 285 mg |


-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 2h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 3h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 3h; Heating; | |
| With water; sodium hydroxide In chloroform | A 5 mg B 1 mg |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 3h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 3h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 3h; Heating; |

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 8h; Heating; |
-
-
54910-48-4
squalene 2,3(S)-oxide

-
A
-
559-70-6
beta-amyrin

-
B
-
30300-37-9, 34079-40-8, 465-02-1
germanicol

-
C
-
545-47-1
lupenol

-
D
-
1059-14-9, 20301-85-3, 64090-86-4, 113829-58-6
taraxasterol

| Conditions | Yield |
|---|---|
| With Arabidopsis thaliana LUP1 Product distribution; Cyclization; Enzymatic reaction; | A 8 % Chromat. B 7 % Chromat. C 38 % Chromat. D 4 % Chromat. |


-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 20℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 86 percent / imidazole; DMAP / dimethylformamide / 7 h / 20 °C 2.1: 94 percent / DMAP; pyridine / 16 h / 20 °C 3.1: 98 percent / TBAF / tetrahydrofuran / 3 h / Heating 4.1: 72 percent / PCC / CH2Cl2 / 0.5 h / 20 °C 5.1: p-toluenesulfonylhydrazine; Na2SO4 / ethanol / 3.5 h / 100 °C 5.2: 14 percent / NaBH3CN; p-toluenesulfonic acid monohydrate / dimethylformamide; tetrahydrothiophene 1,1-dioxide / 1 h / 100 °C 6.1: 70 percent / K2CO3 / methanol / 13 h / 50 °C View Scheme | |
| Multi-step reaction with 5 steps 1: pyridine; dmap / N,N-dimethyl-formamide / Reflux 2: pyridine / chloroform / 48 h / 0 - 20 °C 3: iron(III) chloride / tetrahydrofuran / 48 h / Reflux 4: pyridinium chlorochromate / dichloromethane / 0.5 h / 20 °C 5: hydrazine hydrate; potassium hydroxide / diethylene glycol / 6 h / Reflux View Scheme |
-
-
27570-20-3
betulin monoacetate

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 72 percent / PCC / CH2Cl2 / 0.5 h / 20 °C 2.1: p-toluenesulfonylhydrazine; Na2SO4 / ethanol / 3.5 h / 100 °C 2.2: 14 percent / NaBH3CN; p-toluenesulfonic acid monohydrate / dimethylformamide; tetrahydrothiophene 1,1-dioxide / 1 h / 100 °C 3.1: 70 percent / K2CO3 / methanol / 13 h / 50 °C View Scheme |
-
-
501124-70-5
(3β)-28-[(dimethylethyl)dimethylsilyloxy]lup-20(29)-en-3-ol

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 94 percent / DMAP; pyridine / 16 h / 20 °C 2.1: 98 percent / TBAF / tetrahydrofuran / 3 h / Heating 3.1: 72 percent / PCC / CH2Cl2 / 0.5 h / 20 °C 4.1: p-toluenesulfonylhydrazine; Na2SO4 / ethanol / 3.5 h / 100 °C 4.2: 14 percent / NaBH3CN; p-toluenesulfonic acid monohydrate / dimethylformamide; tetrahydrothiophene 1,1-dioxide / 1 h / 100 °C 5.1: 70 percent / K2CO3 / methanol / 13 h / 50 °C View Scheme |
-
-
501124-71-6
3-acetoxy-28-tert-butyldimethylsiloxybetulin

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 98 percent / TBAF / tetrahydrofuran / 3 h / Heating 2.1: 72 percent / PCC / CH2Cl2 / 0.5 h / 20 °C 3.1: p-toluenesulfonylhydrazine; Na2SO4 / ethanol / 3.5 h / 100 °C 3.2: 14 percent / NaBH3CN; p-toluenesulfonic acid monohydrate / dimethylformamide; tetrahydrothiophene 1,1-dioxide / 1 h / 100 °C 4.1: 70 percent / K2CO3 / methanol / 13 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8 percent / KOH / bis-(2-hydroxy-ethyl) ether / 2 h / Heating 2: 50 percent / KOH / bis-(2-hydroxy-ethyl) ether / 160 °C / Heating; reflux 2 h, cooled, dil. HCl, ether extr., washed: water, dried, solvent distillation, chromatographed, petrol and benzene:petrol (2:3) elution View Scheme | |
| Multi-step reaction with 2 steps 1: 8 percent / KOH / bis-(2-hydroxy-ethyl) ether / 2 h / Heating 2: 50 percent / KOH / bis-(2-hydroxy-ethyl) ether / 2 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; ethanol for 4h; Heating; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In methanol at 39℃; for 4h; | A 1.7 mg B n/a |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In methanol at 39℃; for 4h; | A 1.3 mg B n/a |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 20℃; for 0.25h; |


| Conditions | Yield |
|---|---|
| With dmap In dichloromethane for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydrogencarbonate In dichloromethane; water | 97.6% |
| With hydrogenchloride; sodium hydrogencarbonate In dichloromethane; water | 97.6% |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 86h; | 97.1% |
-
-
545-47-1
lupenol

-
-
1056441-97-4
2,3,4,6-tetra-O-benzoyl-α,β-D-idopyranosyl trichloroacetimidate

-
-
1620782-32-2
3β-(2,3,4,6-tetra-O-benzoyl-α-D-idopyranosyloxy)lup-20(29)-ene

| Conditions | Yield |
|---|---|
| Stage #1: lupenol; 2,3,4,6-tetra-O-benzoyl-α,β-D-idopyranosyl trichloroacetimidate With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.5h; Molecular sieve; Stage #2: With triethylamine In dichloromethane | 97% |

| Conditions | Yield |
|---|---|
| In cyclohexane for 24h; Reflux; | 96.8% |
| With pyridine; dmap for 12h; Heating; | 63% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 18h; Acetylation; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine for 15h; Addition; Heating; | 95% |
| In cyclohexane for 24h; Reflux; | 81.6% |
-
-
545-47-1
lupenol

-
-
183901-63-5
2,3,4,6-tetra-O-benzoyl-α-D-mannopyranosyl trichloroacetimidate

-
-
1030385-77-3
3β-O-(2,3,4,6-tetra-O-benzoyl-α-D-mannopyranosyl)-lup-20(29)-ene

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; MS 4 Angstroem In dichloromethane at -40℃; for 0.333333h; | 95% |
| Stage #1: lupenol; 2,3,4,6-tetra-O-benzoyl-α-D-mannopyranosyl trichloroacetimidate In dichloromethane at 20℃; for 0.5h; Molecular sieve 4A; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.333333h; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine for 15h; Addition; Heating; | 93% |

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-tri-O-benzoyl-α,β-L-arabinopyranosyl trichloroacetimidate; lupenol In dichloromethane at 20℃; for 0.333333h; Molecular sieve; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.5h; Molecular sieve; | 93% |

| Conditions | Yield |
|---|---|
| With Jones reagent; acetic acid In chloroform at 20℃; for 0.166667h; Jones Oxidation; Cooling with ice; | 91% |
| With pyridinium chlorochromate In dichloromethane; isopropyl alcohol at 20℃; | 85% |
| With pyridinium chlorochromate In dichloromethane at 20℃; for 24h; | 80% |
-
-
545-47-1
lupenol

-
-
108-24-7
acetic anhydride

-
-
1617-68-1, 6610-54-4, 55870-40-1, 74345-30-5, 101627-42-3
lupeol acetate

| Conditions | Yield |
|---|---|
| With pyridine; dmap Reflux; | 91% |
| With dmap In dichloromethane for 3h; | 82% |
| With pyridine for 0.0333333h; microwave irradiation; | 79% |
-
-
545-47-1
lupenol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
117562-62-6
lupeol 3-O-(2',3',4',6'-tetra-O-acetyl-β-D-glucopyranoside)

| Conditions | Yield |
|---|---|
| With mercury(II) cyanide In acetonitrile at 90℃; for 1h; | 88% |
-
-
545-47-1
lupenol


-
-
1269649-40-2
lupeol 2,3,4-tri-O-benzoyl-β-D-xylopyranosyl-(1->3)-[2,3,4-tri-O-acetyl-α-L-rhamnopyransyl-(1->2)]-4,6-di-O-benzylidene-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.666667h; Inert atmosphere; | 87% |

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-tri-O-benzoyl-α,β-L-arabinopyranosyl trichloroacetimidate; lupenol In dichloromethane at 20℃; for 0.333333h; Molecular sieve; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.5h; Molecular sieve; | A 86% B 6% |


| Conditions | Yield |
|---|---|
| With lithium; ethylenediamine for 2h; Heating; | 85% |
| With acetic acid; platinum at 60℃; Hydrogenation; | |
| With platinum(IV) oxide; hydrogen; acetic acid at 60 - 70℃; |

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 85% |

| Conditions | Yield |
|---|---|
| With cesium fluoride; sodium hydroxide In dimethyl sulfoxide at 130℃; for 2.5h; | 76% |
-
-
4160-82-1
3,3-dimethylglutaric anhydride

-
-
545-47-1
lupenol

-
-
1442461-21-3
3{beta}-O-(3',3'-dimethylglutaryl)lupeol

| Conditions | Yield |
|---|---|
| In cyclohexane for 24h; Reflux; | 75.8% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 0.5h; | 72% |
-
-
545-47-1
lupenol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
A
-
117584-55-1
A-nor-Δ3,4-lupeol

-
B
-
117562-62-6
lupeol 3-O-(2',3',4',6'-tetra-O-acetyl-β-D-glucopyranoside)

| Conditions | Yield |
|---|---|
| With cadmium(II) carbonate In toluene for 2h; Heating; presence of moleculare sieve 4 Angstroem in Soxhlet apparatus; | A 6% B 70% |

-
-
545-47-1
lupenol

-
-
64181-07-3
3β-hydroxy-lup-20(29)-en-30-al

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In ethanol at 20℃; for 48h; Reflux; | 70% |
| With selenium(IV) oxide In 1,4-dioxane; water for 12h; Reflux; | 60% |
| With selenium(IV) oxide In ethanol for 18h; Reflux; | 54% |
| With selenium(IV) oxide In ethanol for 48h; Reflux; | 45% |
| With selenium(IV) oxide In ethanol for 24h; Reflux; | 44% |
-
-
1079340-44-5
3,3-dimethyl succinic anhydride

-
-
545-47-1
lupenol

| Conditions | Yield |
|---|---|
| With pyridine; dmap Reflux; | 70% |

| Conditions | Yield |
|---|---|
| In cyclohexane for 24h; Reflux; | 68.9% |
-
-
2938-48-9
3,3-dimethyl glutaric acid anhydride

-
-
545-47-1
lupenol

-
-
1442461-23-5
3{deta}-O-(4',4'-dimethylglutaryl)lupeol

| Conditions | Yield |
|---|---|
| In cyclohexane for 24h; Reflux; | 68.6% |
-
-
545-47-1
lupenol

-
-
20329-98-0, 20329-99-1, 90-50-6
(E)-3,4,5-trimethoxy-cinnamic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 65.3% |
Lupeol Chemical Properties
Molecular structure of Lup-20(29)-en-3-ol, (3b)- (CAS NO.545-47-1) is:
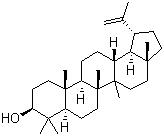
Product Name: Lup-20(29)-en-3-ol, (3b)-
CAS Registry Number: 545-47-1
IUPAC Name: (1R,3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a,5a,5b,8,8,11a-hexamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysen-9-ol
Molecular Weight: 426.7174 [g/mol]
Molecular Formula: C30H50O
XLogP3-AA: 9.9
H-Bond Donor: 1
H-Bond Acceptor: 1
Storage temp.: 2-8 °C
Surface Tension: 34.2 dyne/cm
Density: 0.977 g/cm3
Flash Point: 216.9 °C
Enthalpy of Vaporization: 86.89 kJ/mol
Boiling Point: 488.1 °C at 760 mmHg
Vapour Pressure: 1.41E-11 mmHg at 25 °C
EINECS: 208-889-9
Product Categories: Tri-Terpenoids
Lupeol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | > 2gm/kg (2000mg/kg) | Fitoterapia. Vol. 68, Pg. 9, 1997. | |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Fitoterapia. Vol. 68, Pg. 9, 1997. |
Lupeol Safety Profile
WGK Germany: 2
RTECS: OK5763000
Lupeol Specification
Lup-20(29)-en-3-ol, (3b)- , its cas register number is 545-47-1. It also can be called Lupeol ; (3beta)-Lup-20(29)-en-3-ol ; Fagarasterol .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View