-
Name
Miconazole
- EINECS 245-324-5
- CAS No. 22916-47-8
- Article Data6
- CAS DataBase
- Density 1.4 g/cm3
- Solubility Freely soluble in alcohols or acetone. Also soluble in DMF or chloroform. Insoluble in water
- Melting Point 159-163 °C
- Formula C18H14Cl4N2O
- Boiling Point 555.1 °C at 760 mmHg
- Molecular Weight 416.134
- Flash Point 289.5 °C
- Transport Information
- Appearance White powder.
- Safety 22-36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Imidazole,1-[2,4-dichloro-b-[(2,4-dichlorobenzyl)oxy]phenethyl]-(8CI);Daktanol;DaktarinIV;Florid-F;Lauriad;MJR 1762;Miconazole;Monistat IV;NSC 170986;R 18134;Zimybase;
- PSA 27.05000
- LogP 6.45480
Synthetic route
-
-
94-99-5
2,4-Dichlorobenzyl chloride

-
-
24155-42-8
1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Substitution; Stage #2: 2,4-Dichlorobenzyl chloride In tetrahydrofuran at 45℃; for 16h; Substitution; | 73% |
| Stage #1: 1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol With sodium hydride In N,N-dimethyl-formamide Inert atmosphere; Stage #2: 2,4-Dichlorobenzyl chloride In N,N-dimethyl-formamide at 20℃; |
-
-
874-42-0
2,4-dichlorobenzaldeyhde

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 82 percent / aq. KOH / acetonitrile / 3 h / 60 °C 2.1: 63 percent / pyridine / ethanol / 12 h / Heating 3.1: NaH; HMPA / tetrahydrofuran / 0.5 h / 20 °C 3.2: 73 percent / tetrahydrofuran / 16 h / 45 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / acetonitrile / 60 - 65 °C / Inert atmosphere 2.1: sodium hydride / N,N-dimethyl-formamide / Inert atmosphere 2.2: 20 °C / Inert atmosphere 3.1: sodium hydride / N,N-dimethyl-formamide / Inert atmosphere 3.2: 20 °C View Scheme |
-
-
13692-15-4
2-(2,4-dichlorophenyl)oxirane

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 63 percent / pyridine / ethanol / 12 h / Heating 2.1: NaH; HMPA / tetrahydrofuran / 0.5 h / 20 °C 2.2: 73 percent / tetrahydrofuran / 16 h / 45 °C View Scheme |

-
-
24155-42-8
1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 2h; Stage #2: 2,4-dichlorobenzyl methanesulfonate In N,N-dimethyl-formamide at 20℃; for 12h; | 118 mg |
-
-
1777-82-8
(2,4-dichlorophenyl)methanol

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / dichloromethane / 2 h / 0 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 2 h / 0 °C 2.2: 12 h / 20 °C View Scheme |
-
-
4252-78-2
2,2',4'-trichloroacetophenone

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: dichloromethane / 12 h / 0 - 20 °C 2.1: sodium tetrahydroborate; methanol / dichloromethane / 2 h / 20 °C 3.1: sodium hydride / N,N-dimethyl-formamide / 2 h / 0 °C 3.2: 12 h / 20 °C View Scheme |
-
-
46503-52-0
1-(2,4-dichlorophenyl)-2-(imidazol-1-yl)ethanone

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium tetrahydroborate; methanol / dichloromethane / 2 h / 20 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 2 h / 0 °C 2.2: 12 h / 20 °C View Scheme |
-
-
150994-84-6
4-((1,3-bis(dodecanoyloxy)propan-2-yl)oxy)-4-oxobutanoic acid

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 6h; | 100% |
-
-
112-82-3
hexadecanyl bromide

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 95% |
-
-
111-83-1
1-bromo-octane

-
-
22916-47-8
miconazole

-
-
57264-59-2
1-(2-((2,4-dichlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-3-octyl-1H-imidazol-3-ium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 93% |
| In acetonitrile for 2h; Reflux; | 93% |

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 92% |
| In ethanol; water at 20℃; for 24h; | 82% |
-
-
111-25-1
1-bromo-hexane

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 90% |
-
-
143-15-7
1-dodecylbromide

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| In methanol for 3h; Reflux; | 86.96% |
-
-
141776-91-2
1-bromomethyl-3,5-difluoro-benzene

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethyl acetate for 48h; Heating; | 86% |
-
-
112-29-8
1-bromo dodecane

-
-
22916-47-8
miconazole

-
-
57264-60-5
3-decyl-1-(2-((2,4-dichlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazol-3-ium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 85% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of copper compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 85% |
-
-
112-71-0
1-Bromotetradecane

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 85% |
-
-
26042-63-7
silver(I) hexafluorophosphate

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In methanol; chloroform for 3h; Reflux; | 83% |


-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 24h; | 82% |
-
-
14104-20-2
silver tetrafluoroborate

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 24h; | 82% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of zinc compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 80% |

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 80% |

| Conditions | Yield |
|---|---|
| In methanol; chloroform for 5h; Reflux; | 77% |

-
-
22916-47-8
miconazole

-
-
1410795-51-5
[Mn(II)Cl2(miconazol)2(water)2]*water

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of manganese compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 75% |

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,2-dimethoxyethane at 20℃; for 5h; Inert atmosphere; Schlenk technique; | 73% |
-
-
22916-47-8
miconazole

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 12h; | 71% |
| In ethyl acetate at 20℃; for 48h; | 69% |
| Heating; Yield given; |

| Conditions | Yield |
|---|---|
| In methanol; chloroform at 20℃; for 0.075h; Reflux; | 71% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of chromium compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 70% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of cobalt compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 68% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of iron compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 65% |

| Conditions | Yield |
|---|---|
| In acetone for 20h; Inert atmosphere; Schlenk technique; | 65% |

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 24h; | 65% |

| Conditions | Yield |
|---|---|
| In ethanol; water addn. of hot soln. of nickel compd. in ethanol/water (1:1) to hot ethanolic soln. of miconazol at 60°C, stirring at reflux for 1 h; filtration, washing with ethanol/water and petroleum ether, elem. anal.; | 60% |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Heating; | 52% |

-
-
22916-47-8
miconazole

| Conditions | Yield |
|---|---|
| Stage #1: MnBr(bpyCOOCH3,COOCH3)(CO)3 With silver trifluoromethanesulfonate In acetone at 20℃; for 3h; Darkness; Inert atmosphere; Schlenk technique; Stage #2: miconazole at 20℃; for 20h; Schlenk technique; Inert atmosphere; Darkness; Stage #3: ammonium hexafluorophosphate In methanol; water Schlenk technique; Inert atmosphere; Darkness; | 49% |

MICONAZOLE Chemical Properties
MF: C18H14Cl4N2O
MW: 416.13
EINECS: 245-324-5
Mol File: 22916-47-8.mol
mp: 159-163°C
Synonyms:1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-imidazol;1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-imidazol;1-[2-[(2,4-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole;Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-;Imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-;micronazol;Minostate;mjr1762
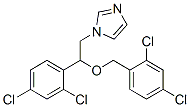
The structure of Miconazole(22916-47-8) is:
MICONAZOLE Uses
2、Miconazole(22916-47-8) is also used in Ektachrome film developing (along with surfactants such as Photo-Flo) in the final rinse of the Kodak E-6 process and similar Fuji CR-56 process, replacing formaldehyde.
MICONAZOLE Toxicity Data With Reference
| 1. | sln-asn 410 µg/L | MUREAV Mutation Research. 79 (1980),169. | ||
| 2. | ivn-inf TDLo:104 mg/kg/4D-I | MJAUAJ Medical Journal of Australia. 146 (1987),57. | ||
| 3. | ipr-rat LD50:349 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 31 (1981),2145. | ||
| 4. | orl-mus LD50:872 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 31 (1981),2145. | ||
| 5. | ipr-mus LD50:451 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 31 (1981),2145. | ||
| 6. | ivn-dog LD50:60 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 19 (1980),7. | ||
| 7. | ivn-mam LD50:100 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 19 (1980),7. |
MICONAZOLE Safety Profile
Risk Statements: Harmful if swallowed
Safety Statements: Wear suitable protective clothing
RIDADR: 3249
HazardClass: 6.1(b)
PackingGroup: III
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Cl− and NOx.
MICONAZOLE Specification
Storage:Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling:Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
2、Fire Fighting
Wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Extinguishing media: Use agent most appropriate to extinguish fire. In case of fire use water spray, dry chemical, carbon dioxide, or appropriate foam.
Related Products
- MICONAZOLE
- Miconazole nitrate
- 229177-52-0
- 229177-79-1
- 22918-01-0
- 229180-64-7
- 22919-26-2
- 22919-46-6
- 22919-49-9
- 22921-58-0
- 2292-16-2
- 22921-68-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View