-
Name
Methyl glycolate
- EINECS 202-502-7
- CAS No. 96-35-5
- Article Data109
- CAS DataBase
- Density 1.14 g/cm3
- Solubility Miscible with water.
- Melting Point 149-151 °C
- Formula C3H6O3
- Boiling Point 151.099 °C at 760 mmHg
- Molecular Weight 90.0788
- Flash Point 67.222 °C
- Transport Information
- Appearance Clear colorless liquid
- Safety 23-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Methyl hydroxyacetate;NSC 27786;Acetic acid, hydroxy-, methyl ester;Glycolic acid, methyl ester (8CI);
- PSA 46.53000
- LogP -0.84830
Synthetic route

| Conditions | Yield |
|---|---|
| With C33H32Cl2N2P2Ru; hydrogen; sodium methylate In para-xylene; toluene at 5 - 100℃; under 37503.8 Torr; for 4h; Reagent/catalyst; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| With (o-PPh2C6H4NH2)[EtNH(CH2)2NHEt]RuCl2; hydrogen; sodium methylate In tetrahydrofuran; para-xylene at 5 - 100℃; under 7500.75 Torr; for 1.5h; Reagent/catalyst; Pressure; Temperature; Glovebox; | 99% |
| With hydrogen In methanol at 219.84℃; under 18751.9 Torr; Temperature; Reagent/catalyst; Autoclave; | 32% |
| With 1,1,1-tris(n-butylthiomethyl)ethane; hydrogen; zinc; Λ(+)-tris(pentane-2,5-dionato)ruthenium In methanol at 100℃; under 60004.8 Torr; for 69h; Kinetics; Product distribution; Further Variations:; Reagents; |

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; carbon monoxide With 1,3,5-Trioxan; methanesulfonic acid; phosphoric acid In dichloromethane; benzene at 150℃; under 54005.4 Torr; Stage #2: methanol In dichloromethane; benzene at 190℃; under 9000.9 Torr; for 24h; Temperature; Reagent/catalyst; Solvent; Pressure; | 97.4% |
| Stage #1: formaldehyd; carbon monoxide With 4-n-butyl-4-(3-sulfopropyl)thiomorpholinium 1,1-dioxide trifluoromethane sulfonate In sulfolane at 160℃; under 37503.8 Torr; for 8h; Autoclave; Stage #2: methanol With 4-n-butyl-4-(3-sulfopropyl)thiomorpholinium 1,1-dioxide trifluoromethane sulfonate In sulfolane at 80℃; for 2h; Catalytic behavior; Reagent/catalyst; Solvent; Pressure; Time; Temperature; Concentration; Autoclave; | 92% |
| Stage #1: formaldehyd; carbon monoxide In sulfolane at 200℃; under 60006 Torr; Autoclave; Stage #2: methanol With nitric acid for 4h; Reflux; chemoselective reaction; | 47.7 %Chromat. |


| Conditions | Yield |
|---|---|
| With amberlyst-15 for 16h; | 96% |
| With toluene-4-sulfonic acid for 10h; Reflux; | 87.2% |
| With boric acid at 20℃; for 18h; | 80% |
-
-
146351-72-6
[(1,1-dimethylethyl)(dimethyl)silyloxy]acetic acid methyl ester

-
-
96-35-5
glycolic acid methyl ester

| Conditions | Yield |
|---|---|
| With formic acid In water; acetonitrile at 20℃; | 95% |
-
-
553-90-2
Dimethyl oxalate

-
-
67-56-1
methanol

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| With hydrogen at 199.84℃; under 22502.3 Torr; Reagent/catalyst; | A 90.6% B n/a |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 10h; Reflux; | A n/a B 87.2% |


| Conditions | Yield |
|---|---|
| With C32H32Cl2N2P2Ru; hydrogen; sodium methylate In para-xylene; toluene at 5 - 100℃; under 37503.8 Torr; for 4h; Reagent/catalyst; Glovebox; | A 86% B 13% |
| With hydrogen In methanol at 219.84℃; under 18751.9 Torr; Autoclave; | A 76% B n/a |
| With hydrogen In methanol at 219.84℃; under 18751.9 Torr; Autoclave; | A 31% B n/a |

| Conditions | Yield |
|---|---|
| With zeolite Sn-MFI for 18h; Reagent/catalyst; Time; Sealed tube; Green chemistry; | 83% |

| Conditions | Yield |
|---|---|
| With hydrogen; <(Ph3P)(Ph2P)RuH2-K+*diglyme>2 In toluene at 90℃; under 4650.4 Torr; for 20h; Title compound not separated from byproducts; | A n/a B 70% |
| With (K(1+))2<(Ph3P)3(Ph2P)Ru2H4>(2-)*2C6H14O3; hydrogen In toluene at 90℃; under 4650.4 Torr; for 20h; |
-
-
67-56-1
methanol

-
-
141-46-8
Glycolaldehyde

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| 1-hydroxytetraphenylcyclopentadienyl(tetraphenyl-2,4-cyclopentadien-1-one)-μ-hydrotetracarbonyldiruthenium(II) In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B 50% |
| [RuH(OCOMe)(PPh3)3] In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B 35% |
| dihydridotris(triphenylphosphine)ruthenium In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B 35% |

-
-
110-88-3
1,3,5-Trioxan

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
107-31-3
Methyl formate

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-Trioxan; carbon monoxide With 1-methyl-3-(4-sulfonylbutyl)-1H-imidazol-3-ium trifluoromethanesulfonate; water In tetrahydrofuran at 170℃; under 37503.8 Torr; for 8h; Autoclave; Stage #2: methanol In tetrahydrofuran at 80℃; for 2h; Autoclave; | A 49.9% B 37.9% |


| Conditions | Yield |
|---|---|
| With Au NCs/TiO2; oxygen; potassium carbonate; molybdenum(VI) oxide at 119.84℃; under 7500.75 Torr; for 4h; Catalytic behavior; Time; Temperature; | 39.2% |

| Conditions | Yield |
|---|---|
| With Au NCs/TiO2; oxygen; potassium carbonate; molybdenum(VI) oxide at 119.84℃; under 7500.75 Torr; for 4h; Catalytic behavior; Time; | 38.7% |
-
-
67-56-1
methanol

-
-
141-46-8
Glycolaldehyde

-
A
-
30934-97-5
2,2-dimethoxyethanol

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 35% |
| With triethylamine; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 35% |
| With potassium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 20℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 27% |
| With caesium carbonate; tris(triphenylphosphine)ruthenium(II) chloride In 1,4-dioxane at 20℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 27% |
| With potassium hydroxide; dichloro(pentamethylcyclopentadienyl) iridium In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; | A n/a B n/a C 20% |


| Conditions | Yield |
|---|---|
| In quinoline at 20℃; for 3h; | 34% |

| Conditions | Yield |
|---|---|
| With Au NCs/TiO2; oxygen at 99.84℃; under 7500.75 Torr; for 2h; | 30.4% |
| [Cp*Ru(OMe)2] In 1,4-dioxane at 70℃; for 2 - 5h; Product distribution / selectivity; |
-
-
110-88-3
1,3,5-Trioxan

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
107-31-3
Methyl formate

-
C
-
6290-49-9
methyl methoxyacetate

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-Trioxan; carbon monoxide With 1-methyl-3-(4-sulfonylbutyl)-1H-imidazol-3-ium trifluoromethanesulfonate; water In toluene at 170℃; under 37503.8 Torr; for 8h; Autoclave; Stage #2: methanol In toluene at 80℃; for 2h; Autoclave; | A 15.4% B 23.9% C 18.1% |

-
-
553-90-2
Dimethyl oxalate

-
A
-
67-56-1
methanol

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
64-17-5
ethanol

-
D
-
79-20-9
acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogen at 199.84℃; under 18751.9 Torr; Catalytic behavior; Reagent/catalyst; Autoclave; | A n/a B n/a C 14.2% D n/a |

-
-
105157-41-3
C6H11ClN2O4

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
7541-16-4
methyl N,N-dimethylcarbamate

-
C
-
105157-38-8
C6H12N2O4

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 0℃; for 0.75h; Yields of byproduct given; | A n/a B n/a C 14.1% |

-
-
67-56-1
methanol

-
-
131543-46-9
Glyoxal

-
A
-
79-14-1
glycolic Acid

-
B
-
2517-44-4
1,2-dimethoxy 1,2-dimethoxy-ethane

-
C
-
96-35-5
glycolic acid methyl ester

-
D
-
51673-84-8
dimethoxyacetaldehyde

| Conditions | Yield |
|---|---|
| KA-3 for 4h; | A n/a B 11% C n/a D n/a |

-
-
553-90-2
Dimethyl oxalate

-
-
67-56-1
methanol

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
34557-54-5
methane

| Conditions | Yield |
|---|---|
| With hydrogen at 473℃; under 22502.3 Torr; | A 6.8% B n/a |


| Conditions | Yield |
|---|---|
| With sulfuric acid; water at 210 - 260℃; under 588406 - 661957 Torr; CO-Druck; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With amberlyst wet 15 at 130℃; |
-
-
67-56-1
methanol

-
-
590-29-4
potassium formate

-
-
96-34-4
methyl chloroacetate

-
-
96-35-5
glycolic acid methyl ester

| Conditions | Yield |
|---|---|
| at 110 - 115℃; |


| Conditions | Yield |
|---|---|
| With methanol; glycolacidic sodium at 160℃; im Druckrohr; |
-
-
67-56-1
methanol

-
-
683-51-2
2-chloro-2-propenal

-
A
-
553-90-2
Dimethyl oxalate

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
89-91-8
Methyl dimethoxyacetate

-
D
-
96-34-4
methyl chloroacetate

-
E
-
298-12-4
Glyoxilic acid

| Conditions | Yield |
|---|---|
| With ozone at -78℃; Product distribution; ozonolysis; | A 18 % Spectr. B 14 % Spectr. C 44 % Spectr. D 1 % Spectr. E 18 % Spectr. |

-
-
67-56-1
methanol

-
-
683-70-5
3-chloro-but-3-en-2-one

-
A
-
553-90-2
Dimethyl oxalate

-
B
-
96-35-5
glycolic acid methyl ester

-
C
-
79-20-9
acetic acid methyl ester

-
D
-
10076-48-9
methyl 2,2-dimethoxypropionate

-
E
-
96-34-4
methyl chloroacetate

| Conditions | Yield |
|---|---|
| With ozone at -78℃; Product distribution; ozonolysis; |

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
96-34-4
methyl chloroacetate

-
A
-
96-35-5
glycolic acid methyl ester

-
B
-
79-20-9
acetic acid methyl ester

-
C
-
6290-49-9
methyl methoxyacetate

-
D
-
108-59-8
malonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| rhodium(III) chloride; KI/C at 150℃; for 4h; Product distribution; also other mono-substituted methyl halides; | A 0.1 % Chromat. B 0.9 % Chromat. C 0.1 % Chromat. D 10.4 % Chromat. |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 100% |
| In pyridine at 20℃; for 12h; | 91% |
-
-
96-35-5
glycolic acid methyl ester

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
154698-92-7
methyl 2-((tert-butyl(diphenyl)silyl)oxy)acetate

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In N,N-dimethyl-formamide for 10h; Ambient temperature; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 3h; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 3h; Inert atmosphere; | 98% |
-
-
96-35-5
glycolic acid methyl ester

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
146351-72-6
[(1,1-dimethylethyl)(dimethyl)silyloxy]acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide for 1.5h; Ambient temperature; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide for 90h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With water at 95 - 97℃; under 760.051 Torr; for 2h; Reactive distillation; | 100% |
| With water; Candida rugosa lipase In aq. phosphate buffer at 45℃; for 30h; pH=7.2; Enzymatic reaction; | |
| With water In methanol at 100℃; |

| Conditions | Yield |
|---|---|
| With hydrazine In methanol at 20℃; for 74h; Heating / reflux; | 100% |
| With hydrazine hydrate In ethanol at 20℃; for 3h; Reflux; | 80% |
| With hydrazine hydrate In methanol for 8h; Reflux; | |
| With hydrazine hydrate In methanol for 8h; Reflux; | |
| With hydrazine hydrate In methanol at 60℃; for 96h; Inert atmosphere; |
-
-
288-32-4
1H-imidazole

-
-
96-35-5
glycolic acid methyl ester

-
-
146351-72-6
[(1,1-dimethylethyl)(dimethyl)silyloxy]acetic acid methyl ester

| Conditions | Yield |
|---|---|
| In dimethylformamide [DMF]; water | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 96h; | 100% |
-
-
10431-98-8
2-ethyl-4,5-dihydrooxazole

-
-
96-35-5
glycolic acid methyl ester

-
-
333-27-7
methyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-4,5-dihydrooxazole; methyl trifluoromethanesulfonate In chlorobenzene at 20 - 110℃; for 0.666667h; Stage #2: glycolic acid methyl ester With 2,6-dimethylpyridine In chlorobenzene at 0 - 20℃; for 18h; | 100% |

-
-
10431-98-8
2-ethyl-4,5-dihydrooxazole

-
-
96-35-5
glycolic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-4,5-dihydrooxazole With methyl trifluoromethanesulfonate In chlorobenzene at 20 - 110℃; for 0.666667h; Stage #2: glycolic acid methyl ester With 2,6-dimethylpyridine In chlorobenzene at 0 - 20℃; for 18h; Product distribution / selectivity; | 100% |
-
-
123-75-1
pyrrolidine

-
-
96-35-5
glycolic acid methyl ester

-
-
45657-97-4
2-hydroxy-1-(pyrrolidin-1-yl)ethanone

| Conditions | Yield |
|---|---|
| at 20℃; for 96h; | 100% |
-
-
67443-38-3
5-bromo-2-chloro-3-nitropyridine

-
-
96-35-5
glycolic acid methyl ester

-
-
227963-72-6
methyl 2-((5-bromo-3-nitropyridin-2-yl)oxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: glycolic acid methyl ester With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: 5-bromo-2-chloro-3-nitropyridine In tetrahydrofuran at 20℃; for 18h; | 99% |
-
-
96-35-5
glycolic acid methyl ester

-
-
1411884-59-7
methyl 4-prenyloxyphenylacetate

-
-
1411884-62-2
4-hydroxy-3-(4-prenyloxyphenyl)furan-2(5H)-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 50h; Inert atmosphere; | 99% |
-
-
96-35-5
glycolic acid methyl ester

-
-
95-50-1
1,2-dichloro-benzene

-
-
6956-85-0
Methyl (2-chlorophenoxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: glycolic acid methyl ester With sodium methylate at 20℃; Stage #2: 1,2-dichloro-benzene at 60℃; | 99% |

| Conditions | Yield |
|---|---|
| With Mo1P3-MCM-48 at 100 - 120℃; for 3h; Reagent/catalyst; Temperature; | 98.3% |
| With catalyst Mo1P3-MCM-48 (molar ratio of Mo1P3 and MCM-48: 25:100) at 100 - 120℃; for 3h; Reagent/catalyst; Temperature; | 66.38% |
-
-
96-35-5
glycolic acid methyl ester

-
-
905589-59-5
(3,4-dimethoxoxyphenyl)methyl 2,2,2-trichloroacetimidate

-
-
185300-91-8
(3,4-Dimethoxy-benzyloxy)-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane for 36h; | 98% |
-
-
96-35-5
glycolic acid methyl ester

-
-
34837-84-8
4-fluorobenzeneacetic acid methyl ester

-
-
100074-46-2
3-(4-fluorophenyl)-4-hydroxyfuran-2(5H)-one

| Conditions | Yield |
|---|---|
| Stage #1: glycolic acid methyl ester; 4-fluorobenzeneacetic acid methyl ester With potassium tert-butylate In tetrahydrofuran for 16h; Dieckmann reaction; Inert atmosphere; Reflux; Stage #2: With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; Cooling; | 98% |
| With potassium tert-butylate In tetrahydrofuran for 8h; Dieckmann Condensation; Reflux; |

| Conditions | Yield |
|---|---|
| With C24H38Cl2N3PRu; hydrogen; sodium methylate In isopropyl alcohol at 100℃; under 38002.6 Torr; for 2h; Autoclave; | 97% |
| With C24H38Cl2N3PRu; hydrogen; sodium methylate In isopropyl alcohol at 100℃; under 37503.8 Torr; for 2h; | 97% |
| With sodium tetrahydroborate In diethylene glycol dimethyl ether at 32 - 35℃; for 2h; | 89% |
-
-
96-35-5
glycolic acid methyl ester

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
23782-85-6
3-phenyltetronic acid

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 16h; Heating; | 97% |
| Stage #1: glycolic acid methyl ester; benzeneacetic acid methyl ester With potassium tert-butylate In tetrahydrofuran for 16h; Dieckmann reaction; Inert atmosphere; Reflux; Stage #2: With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; Cooling; | 97% |
| With potassium tert-butylate In tetrahydrofuran for 16h; Inert atmosphere; Reflux; | 63% |
| With potassium tert-butylate In tetrahydrofuran for 8h; Dieckmann Condensation; Reflux; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; Mitsunobu reaction; | 97% |
-
-
96-35-5
glycolic acid methyl ester

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
88016-31-3
(trifluoromethanesulfonyloxy)acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -78 - 20℃; Inert atmosphere; | 97% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -20℃; for 1h; Inert atmosphere; | 93% |
| In dichloromethane at 0 - 5℃; for 1.5h; Inert atmosphere; | 70% |
| With pyridine In dichloromethane at -10 - 20℃; for 4.5h; Inert atmosphere; | 70% |
| With pyridine In dichloromethane at -10℃; |

| Conditions | Yield |
|---|---|
| With triethylamine In (2S)-N-methyl-1-phenylpropan-2-amine hydrate; dichloromethane | 96% |
-
-
96-35-5
glycolic acid methyl ester

-
-
27798-60-3
methyl 2-(2-methoxyphenyl)acetate

-
-
100074-47-3
4-hydroxy-3-(2-methoxyphenyl)furan-2(5H)-one

| Conditions | Yield |
|---|---|
| Stage #1: glycolic acid methyl ester; methyl 2-(2-methoxyphenyl)acetate With potassium tert-butylate In tetrahydrofuran for 16h; Dieckmann reaction; Inert atmosphere; Reflux; Stage #2: With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; Cooling; | 95% |
| With potassium tert-butylate In tetrahydrofuran for 8h; Dieckmann Condensation; Reflux; |
-
-
96-35-5
glycolic acid methyl ester

-
-
920-46-7
Methacryloyl chloride

-
-
13318-09-7
methacrylic acid 2-methoxy-2-oxoethyl diester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 95% |
| With triethylamine In dichloromethane at 35℃; for 4h; Schlenk technique; Inert atmosphere; Cooling with ice; | 92% |
| With triethylamine In dichloromethane at 0 - 20℃; for 22h; | 78.7% |
-
-
96-35-5
glycolic acid methyl ester

-
-
570391-18-3
3-chloro-5-(trifluoromethyl)phenol

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; Inert atmosphere; | 95% |
Methyl glycolate Specification
Methyl glycolate is an organic compound with the formula C3H6O3, and its systematic name is the same with the product name. With the CAS registry number 96-35-5, it is also named as Methyl hydroxyacetate. It belongs to the product categories of Acids and Derivatives; Alcohols and Derivatives; C2 to C5; Carbonyl Compounds; Esters. Its EINECS number is 202-502-7. In addition, the molecular weight is 90.08. This chemical should be sealed and stored in a cool and ventilated place. Moreover, it should be protected from oxides, heat and fire. It is used in organic synthesis, and it is also used as high-grade cleaning solvent.
Physical properties of Methyl glycolate are: (1)ACD/LogP: -0.902; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.90; (4)ACD/LogD (pH 7.4): -0.90; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 7.70; (8)ACD/KOC (pH 7.4): 7.70; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 46.53 Å2; (13)Index of Refraction: 1.402; (14)Molar Refractivity: 19.257 cm3; (15)Molar Volume: 79.044 cm3; (16)Polarizability: 7.634×10-24cm3; (17)Surface Tension: 35.52 dyne/cm; (18)Density: 1.14 g/cm3; (19)Flash Point: 67.222 °C; (20)Enthalpy of Vaporization: 45.19 kJ/mol; (21)Boiling Point: 151.099 °C at 760 mmHg; (22)Vapour Pressure: 1.4 mmHg at 25°C.
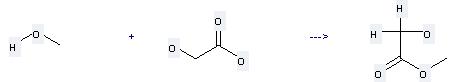
Preparation: this chemical can be prepared by methanol and hydroxyacetic acid at the ambient temperature. This reaction will need reagent thionyl chloride with the reaction time of 48 hours. The yield is about 60%.
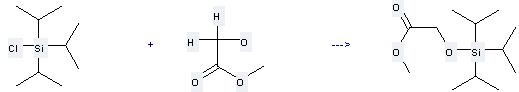
Uses of Methyl glycolate: it can be used to produce triisopropylsilanyloxy-acetic acid methyl ester at the ambient temperature. It will need reagent imidazole and solvent dimethylformamide with the reaction time of 73 hours. The yield is about 72%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. You should not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer). When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)CO
(2)Std. InChI: InChI=1S/C3H6O3/c1-6-3(5)2-4/h4H,2H2,1H3
(3)Std. InChIKey: GSJFXBNYJCXDGI-UHFFFAOYSA-N
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 963-69-9
- 963-74-6
- 963-75-7
- 96-37-7
- 96382-71-7
- 96384-17-7
- 96384-68-8
- 96385-50-1
- 96386-92-4
- 96-40-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View