-
Name
Methylthiouracil
- EINECS 200-252-3
- CAS No. 56-04-2
- Article Data62
- CAS DataBase
- Density 1.36 g/cm3
- Solubility INSOLUBLE
- Melting Point ~330 °C (dec.)(lit.)
- Formula C5H6N2OS
- Boiling Point 342.3 °C at 760 mmHg
- Molecular Weight 142.181
- Flash Point 160.8 °C
- Transport Information
- Appearance white powder
- Safety 36/37-45
- Risk Codes 22-40
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Uracil,6-methyl-2-thio- (8CI);2-Mercapto-4-methyl-6-hydroxypyrimidine;2-Mercapto-6-methyl-4-pyrimidinol;2-Mercapto-6-methylpyrimidyl-4-one;2-Thio-6-methyluracil;4-Hydroxy-2-mercapto-6-methylpyrimidine;4-Methyl-2-thiouracil;6-Methyl-2-thiouracil;6-Methyl-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine;Alkiron;Basecil;Methiacil;Methicil;Methiocil;Muracil;NSC 193526;NSC 9378;Prostrumyl;Thimecil;Thiomidil;Thioryl;Thiothyron;Thiuryl;Thyreostat;Thyreostat I;Tiotiron;USAF-EK 6454;
- PSA 80.74000
- LogP 0.74090
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 125℃; under 15514.9 Torr; for 0.416667h; Biginelli condensation; Microwave irradiation; Inert atmosphere; | 94.6% |
| With potassium hydroxide In ethanol at 80℃; for 5h; | 91% |
| With sodium formate In methanol for 7h; Heating; | 90% |
-
-
72324-39-1
5-acetyl-2,2-dimethyl-1,3-dioxane-4,6-dione

-
-
17356-08-0
thiourea

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| Stage #1: 5-acetyl-2,2-dimethyl-1,3-dioxane-4,6-dione With alcohol Microwave irradiation; Stage #2: thiourea for 0.05h; Microwave irradiation; | 70% |

| Conditions | Yield |
|---|---|
| for 0.0666667h; microwave irradiation; | 62% |
| In dimethylsulfoxide-d6 at 22 - 28℃; Mechanism; | |
| Heating; |
-
-
119730-09-5
2-{[2-(4-bromophenyl)-2-oxoethyl]sulfanyl}-6-methylpyrimidin-4(3H)-one

-
A
-
56-04-2
6-methyl-2-thiouracil


-
C
-
119730-50-6
(E,Z)-2-[2-(4-bromophenyl)-2-oxoethylidene]-6-methyl-2,3-dihydropyrimidin-4(1H)-one

| Conditions | Yield |
|---|---|
| at 180 - 200℃; neat (no solvent); | A 47% B 16% C 9% |

-
-
17649-31-9
6-methyl-2-((2-oxo-2-phenylethyl)thio)pyrimidin-4(3H)-one

-
A
-
56-04-2
6-methyl-2-thiouracil

-
B
-
1380547-50-1
8-benzoyl-4-methyl-7-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)methyl]-6-phenylpyrrolo[1,2-a]pyrimidin-2(1H)-one

-
C
-
119730-48-2
(E,Z)-6-methyl-2-(2-oxo-2-phenylethylidene)-2,3-dyhydropyrimidin-4(1H)-one

| Conditions | Yield |
|---|---|
| at 180 - 200℃; neat (no solvent); | A n/a B 21% C 12% |

-
-
124-41-4
sodium methylate

-
-
141-97-9
ethyl acetoacetate

-
-
17356-08-0
thiourea

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With methanol |


| Conditions | Yield |
|---|---|
| With sodium |

| Conditions | Yield |
|---|---|
| With paraffin oil |
-
-
35523-75-2
7-methyl-5H-1,3,4-thiadiazolo<3,2-a>pirimidin-5-one

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 336h; Ambient temperature; |
-
-
6328-58-1
4-Hydroxy-6-methyl-2-methylthiopyrimidine

-
A
-
3524-87-6
6-methyl-3H-pyrimidin-4-one

-
B
-
626-48-2
6-Methyluracil

-
C
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| In water for 2h; Irradiation; |


-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With formic acid; zinc |

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With sodium ethanolate |

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With sodium amalgam; ethanol | |
| With ethanol; aluminium amalgam | |
| With ammonium hydroxide; iron(II) sulfate | |
| With formic acid; zinc |

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With sodium amalgam; ethanol | |
| With ethanol; aluminium amalgam |

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With ethanol; aluminium amalgam | |
| With acetic acid; phenylhydrazine at 100℃; |

-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 170℃; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; methyloxirane In water at 20℃; | 100% |
| With potassium tert-butylate; iodine In tert-butyl alcohol for 30h; Heating; | 95% |
| With potassium superoxide; 18-crown-6 ether In N,N-dimethyl-formamide for 72h; Ambient temperature; | 79% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
556-56-9
allyl iodid

-
-
62459-06-7
2-allylsulfanyl-6-methylpyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 50℃; Kinetics; Further Variations:; Solvents; Reaction partners; | 99% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
100-39-0
benzyl bromide

-
-
3019-17-8
2-(benzylsulfanyl)-6-methylpyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 50℃; for 0.25h; Kinetics; Further Variations:; Temperatures; Reaction partners; Solvents; | 99% |
| With 1-ethyl-3-methylimidazolium acetate at 65℃; for 3h; regiospecific reaction; |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
121134-48-3
1,1-dibromo-1-decene

-
-
1279713-34-6
(Z)-2-(1-bromodec-1-en-2-ylthio)-6-methylpyrimidin-4(1H)-one

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In water; N,N-dimethyl-formamide at 65℃; for 12h; Inert atmosphere; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With diphosphorus pentasulfide; sodium hydrogencarbonate In diethylene glycol dimethyl ether at 110℃; for 2h; | 98% |
| With tetraphosphorus decasulfide; silica gel for 0.333333h; microwave irradiation; | 40% |
| With tetraphosphorus decasulfide; tetralin at 170℃; | |
| With tetraphosphorus decasulfide; xylene at 155℃; | |
| With tetraphosphorus decasulfide at 200℃; |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
74-88-4
methyl iodide

-
-
6328-58-1
6-methyl-2-methylsulfanyl-3H-pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 4h; | 98% |
| With water; sodium hydroxide at 20℃; for 1h; Cooling with ice; | 86% |
| Stage #1: 6-methyl-2-thiouracil With sodium hydroxide In water for 0.5h; Stage #2: methyl iodide In water at 10 - 20℃; for 24h; | 54% |
| With potassium carbonate In dimethyl sulfoxide | 48% |
| With potassium hydroxide In methanol for 0.166667h; Ambient temperature; |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
21040-45-9
Cinnamyl acetate

-
-
180145-62-4
2-<(E)-cinnamylthio>-6-methyl-4(3H)-pyrimidinone

| Conditions | Yield |
|---|---|
| With palladium diacetate; trisodium tris(3-sulfophenyl)phosphine In water at 60℃; for 20h; | 98% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
7131-09-1
1-(4-(bromomethyl)phenyl)adamantane

-
-
911838-00-1
2-[4-(1-adamantyl)benzylsulfanyl]-6-methyl-pyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 50℃; Kinetics; Further Variations:; Temperatures; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 4h; regioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; iodine In tert-butyl alcohol for 30h; Heating; further halogen as catalysts; | 95% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; iodine In tert-butyl alcohol for 30h; Product distribution; conversion of thiocarbonyl compounds into their corresponding oxygen analogues using alkoxides and hydroxide with halogens as catalysts; | A 95% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: 6-methyl-2-thiouracil With ammonium sulfate; 1,1,1,3,3,3-hexamethyl-disilazane Substitution; Heating; Stage #2: propargyl bromide In acetonitrile for 8h; Substitution; Heating; | 95% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
107-08-4
1-iodo-propane

-
-
62459-04-5
6-methyl-2-propylsulfanylpyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 50℃; Kinetics; Further Variations:; Temperatures; | 94% |
-
-
110-85-0
piperazine

-
-
56-04-2
6-methyl-2-thiouracil

-
-
50-00-0
formaldehyd

-
-
75682-14-3
C-5,N-3-Dipiperazinomethylene-6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| In ethanol; water for 24h; Ambient temperature; | 93.1% |

-
-
56-04-2
6-methyl-2-thiouracil

-
-
106625-69-8
ethyl (E)-3-phenyl-2-propenyl carbonate

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); 1,4-di(diphenylphosphino)-butane In tetrahydrofuran; water at 60℃; for 17h; | 92% |
-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In chloroform at 0℃; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; toluene for 12h; Ambient temperature; | 91% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
21985-04-6
3-phenyl-1-(2-thienyl)-2-propyn-1-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 4h; regioselective reaction; | 91% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
532-27-4
1-chloroacetophenone

-
-
86869-48-9
5-(4-Chloro-phenyl)-3H-thiazol-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water for 2h; Heating; | 90% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
29310-88-1
3-bromoacetylcoumarin

-
-
1233880-54-0
5-methyl-3-[(3'-coumarinyl)]-7H-[1,3]-thiazolo-[3,2-a]-pyrimidine-7-one

| Conditions | Yield |
|---|---|
| at 125℃; for 0.0216667h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With laccase from Agaricus bisporus In aq. phosphate buffer; ethanol at 20℃; for 14h; pH=6; Green chemistry; Enzymatic reaction; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 50 - 60℃; for 2h; complex formation; | 89% |
-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane addn. of 1 equiv. ligand (in MeOH) to Zn-complex (in CH2Cl2), stirring for 5 h; vol. reduction (vac.), crystn. (4 h), collection (filtration), drying (vac.); elem. anal.; | 89% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
88735-43-7
2-(2-bromoacetyl)-3H-benzo[f]chromen-3-one

-
-
1233880-58-4
5-methyl-3-[(5,6-benzo-(3'-coumarinyl))]-7H-[1,3]-thiazolo-[3,2-a]-pyrimidine-7-one

| Conditions | Yield |
|---|---|
| at 125℃; for 0.04h; Microwave irradiation; | 89% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
119730-17-5
6-methyl-2-((2-(4-nitrophenyl)-2-oxoethyl)thio)pyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 87.78% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Reflux; | 87% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
119730-10-8
2-((2-(4-chlorophenyl)-2-oxoethyl)thio)-6-methylpyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 86.85% |
-
-
56-04-2
6-methyl-2-thiouracil

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 86.27% |
-
-
56-04-2
6-methyl-2-thiouracil

-
-
37746-78-4
4-bromo-trans-crotonic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1.08333h; | 86% |
Methylthiouracil Consensus Reports
Methylthiouracil Specification
The Methylthiouracil, also known as 4-Hydroxy-2-mercapto-6-methylpyrimidine, is an organic compound with the formula C5H6N2OS. It belongs to the product category of Pyrimidine. Its EINECS registry number is 200-252-3. With the CAS registry number 56-04-2, its IUPAC name is 6-methyl-2-sulfanylidene-1H-pyrimidin-4-one. What's more, this chemical is a white powder. It is mainly used as surgical preparation of mild hyperthyroidism, thyroid storm, hyperthyroidism and postoperative treatment.
Physical properties of Methylthiouracil: (1)ACD/LogP: 0.31; (2)ACD/LogD (pH 5.5): 0.31; (3)ACD/LogD (pH 7.4): 0.24; (4)ACD/BCF (pH 5.5): 1.01; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 35.06; (7)ACD/KOC (pH 7.4): 29.79; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)Index of Refraction: 1.638; (11)Molar Refractivity: 37.36 cm3; (12)Molar Volume: 103.9 cm3; (13)Surface Tension: 63 dyne/cm; (14)Density: 1.36 g/cm3; (15)Flash Point: 160.8 °C; (16)Enthalpy of Vaporization: 60.93 kJ/mol; (17)Boiling Point: 342.3 °C at 760 mmHg; (18)Vapour Pressure: 3.83E-05 mmHg at 25°C.
Preparation: this chemical can be prepared by acetoacetic acid ethyl ester and thiourea. This reaction will need 4 min with microwave irradiation. The yield is about 69%.
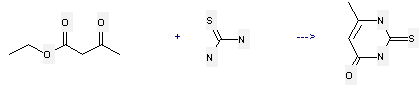
Uses of Methylthiouracil: The drug acts to decrease the formation of stored thyroid hormone, as thyroglobulin in the thyroid gland. The clinical effects of the drug to treat the hyperthyroid state can have a lag period of up to two weeks, depending on the stores of thyroglobulin and other factors.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause damage to health. It may cause inflammation to the skin or other mucous membranes. In addition, it is harmful if swallowed. Whenever you will contact it, please wear suitable protective clothing and gloves. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC1=CC(=O)NC(=S)N1
(2)InChI: InChI=1S/C5H6N2OS/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9)
(3)InChIKey: HWGBHCRJGXAGEU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 200mg/kg (200mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | oral | 2125mg/kg (2125mg/kg) | Pharmaceutical Chemistry Journal Vol. 30, Pg. 320, 1996. | |
| rabbit | LDLo | oral | 2500mg/kg (2500mg/kg) | "Merck Index; an Encyclopedia of Chemicals, Drugs, and Biologicals", 11th ed., Rahway, NJ 07065, Merck & Co., Inc. 1989Vol. 11, Pg. 963, 1989. | |
| rat | LD50 | intraperitoneal | 920mg/kg (920mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Farmaco, Edizione Scientifica. Vol. 13, Pg. 882, 1958. |
| rat | LD50 | oral | 1500mg/kg (1500mg/kg) | Nature. Vol. 157, Pg. 659, 1946. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View