-
Name
N-Carbethoxyphthalimide
- EINECS 245-048-5
- CAS No. 22509-74-6
- Article Data26
- CAS DataBase
- Density 1.398 g/cm3
- Solubility insoluble in water
- Melting Point 90-92 °C
- Formula C11H9NO4
- Boiling Point 353.871 °C at 760 mmHg
- Molecular Weight 219.197
- Flash Point 167.815 °C
- Transport Information
- Appearance White crystalline powder
- Safety 22-24/25-36/37/39-26
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Isoindolinecarboxylicacid, 1,3-dioxo-, ethyl ester (6CI,7CI,8CI);2-(Carbethoxy)phthalimide;Carbethoxyphthalimide;Ethyl 1,3-dioxo-1,3-dihydro-2H-isoindole-2-carboxylate;EthylN-phthaloylcarbamate;N-(Ethoxycarbonyl)phthalimide;
- PSA 63.68000
- LogP 1.37700
Synthetic route
-
-
541-41-3
chloroformic acid ethyl ester

-
-
1074-82-4
potassium phtalimide

-
-
22509-74-6
N-ethoxycarbonylphthalimide

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In toluene at 90℃; for 1h; | 96% |
| With benzene |
-
-
136918-14-4
phthalimide

-
-
541-41-3
chloroformic acid ethyl ester

-
-
22509-74-6
N-ethoxycarbonylphthalimide

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide 1. 0-5 deg C, 90 min; 2. room temperature, 4 h; | 95% |
| With dmap; triethylamine In dichloromethane at -40℃; for 0.0833333h; | 94% |
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; Inert atmosphere; | 94% |
-
-
67-56-1
methanol

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
71964-88-0
ethyl N-<2-(methoxycarbonyl)benzoyl>carbamate

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 20℃; for 2h; | 100% |
| With sodium carbonate In water at 20℃; for 2h; | 100% |
| With sodium hydrogencarbonate In water for 1h; Ambient temperature; | 66% |
| With sodium carbonate In water | 35% |
| With sodium carbonate In water at 20℃; for 5h; |
-
-
71-44-3
Spermine

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
104435-59-8
1,12-diphthalimido-4,9-diazadodecane

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1h; | 100% |
| In dichloromethane Ambient temperature; | 86% |
| In chloroform Ambient temperature; | 70% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
21802-85-7
2-(cyclohexa-1,4-dien-1-yl)ethan-1-amine

-
-
73971-98-9
2-(2-Cyclohexa-1,4-dienyl-ethyl)-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| In toluene 1) 1h ambient temperature 2) 1h reflux; | 100% |
-
-
61-47-2
serotonin creatinine sulfate monohydrate

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
53157-46-3
N,N-phthalimido-2-(5-hydroxy-1H-indole-3-yl)ethylamine

| Conditions | Yield |
|---|---|
| With potassium carbonate In water | 100% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
1204659-78-8
5-amino-3',5'-di-O-(tert-butyldimethylsilyl)-4,5-dihydro-2'-deoxyuridine

-
-
1204659-80-2
2-{1-[4-(tert-butyldimethylsiloxy)-5-(tert-butyldimethylsiloxymethyl)tetrahydrofuran-2-yl]-2,4-dioxohexahydropyrimidin-5-yl}isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| In benzene for 12h; Reflux; Inert atmosphere; | 100% |
-
-
16338-48-0
L-allylglycine

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
110658-33-8
(S)-2-(1,3-dioxoisoindolin-2-yl)pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 21 - 24℃; for 3h; | 99% |
| With triethylamine In tetrahydrofuran for 24h; Inert atmosphere; Schlenk technique; Reflux; | 87% |
| With sodium carbonate In water for 3h; | 65% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
156-87-6
propan-1-ol-3-amine

-
-
883-44-3
N-(3-hydroxypropyl)phthalimide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 6h; Ambient temperature; | 99% |
| In chloroform for 2h; Ambient temperature; Yield given; |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
200402-41-1
(5-amino-pentyl)-(1,1-diethoxy-ethyl)-phosphinic acid ethyl ester

-
-
200402-42-2
N-(5-((1,1-diethoxyethyl)ethoxyphosphoryl)pentyl)phthalimide

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane at 20℃; for 5h; | 99% |
-
-
64-17-5
ethanol

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
71964-89-1
ethyl N-<2-(ethoxycarbonyl)benzoyl>carbamate

| Conditions | Yield |
|---|---|
| for 36h; Heating; | 98% |
-
-
1676-73-9
L-glutamic acid γ-benzyl ester

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
88784-33-2
(2S)-4-benzyloxicarbonyl-2-phthalimidobutanoic acid

| Conditions | Yield |
|---|---|
| With TEA In tetrahydrofuran Heating; | 98% |
| With sodium carbonate |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
56-92-8
histamine dichloride

-
-
5959-80-8
2-(2-(1H-imidazol-4-yl)ethyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 20℃; for 2h; | 98% |
| With sodium carbonate In water at 20℃; for 2h; |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
73-22-3
L-Tryptophan

-
-
32675-71-1, 48208-26-0, 62361-30-2
N-phthalimide-L-tryptophan

| Conditions | Yield |
|---|---|
| Stage #1: L-Tryptophan With sodium carbonate In water Stage #2: N-ethoxycarbonylphthalimide In water at 20℃; for 2h; | 98% |
| With sodium carbonate In water at 20℃; for 1h; | 95% |
| With sodium carbonate In water at 20℃; for 5h; |
-
-
6346-09-4
4-aminobutyrylaldehyde diethylacetal

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
32464-55-4
2-(4,4-diethoxybutyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate for 2h; Ambient temperature; | 97% |
| With triethylamine In tetrahydrofuran for 6h; Ambient temperature; | 95% |
| With triethylamine In tetrahydrofuran at 20℃; for 16h; Cooling with ice; | 94% |
-
-
124-20-9
N-(3-aminopropyl)-1,4-diaminobutane

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
104435-58-7
N1,N10-bisphthaloylspermidine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2.5h; | 96% |
| In dichloromethane at 25℃; for 2h; | 90% |
| In chloroform at 20 - 25℃; for 0.75h; | 75% |
| at 20 - 25℃; | |
| In chloroform Acylation; |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
133-10-8
sodium p-aminosalicylate

-
-
36467-52-4
2-hydroxy-4-(1,3-dioxoisoindolin-2-yl)benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; triethylamine In water at 5℃; for 5h; | 95.1% |
-
-
2432-99-7
11-aminoundecanoic acid

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
4403-42-3
11-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-undecanoic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In water for 6h; | 95% |
| With sodium carbonate In water at 20℃; for 6h; | 93% |
| With sodium carbonate In water at 25℃; for 1h; | 63% |
| 56% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
374808-53-4
1-(2,5-dimethoxy-4-(6-hydroxyhexyl)phenyl)-2-aminopropane

-
-
374808-54-5
6-(2,5-dimethoxy-4-(2-[N,N-phthalimido]propyl)phenyl)hexanol

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; water at 20℃; for 18h; | 95% |
-
-
873581-12-5
tert-butyl (S)-2-amino-6-hydroxyhexanoate

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
873581-13-6
C18H23NO5

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 2h; | 95% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 2h; |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
186828-00-2
(2S,4S)-2-Amino-4-methyl-pentanedioic acid 1-tert-butyl ester

-
-
186828-01-3
(2S,4S)-2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-4-methyl-pentanedioic acid 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrahydrofuran; water for 18h; Ambient temperature; | 94% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
2038-57-5
3-Phenylpropan-1-amine

-
-
54981-86-1
2-(3-phenylpropyl)-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 20℃; | 94% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
188965-96-0
N-[4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-butyl]-2,2,2-trifluoro-acetimidic acid

| Conditions | Yield |
|---|---|
| With TEA In tetrahydrofuran Heating; | 93% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
188965-96-0
N-[4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-butyl]-2,2,2-trifluoro-acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 12h; Heating; | 93% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
2491-18-1
(S)-methyl 2-amino-4-(methylthio)butanoate hydrochloride

-
-
39739-05-4
methyl (S)-2-phthalimido-4-methylthiobutanoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In chloroform; water at 25℃; for 2h; | 92% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
141040-22-4
trans-(3R,4S)-1-(4-methoxyphenyl)-3-amino-4-<(1'S)-1',2'-O-isopropylideneethyl>-2-azetidinone

-
-
141040-23-5
trans-(3R,4S)-1-(4-methoxyphenyl)-3-phthalimido-4-<(1'S)-1',2'-O-isopropylideneethyl>-2-azetidinone

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrahydrofuran; water for 1h; Ambient temperature; | 92% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
1402603-95-5
trans-3-amino-3-(4-bromophenyl)-1-cyclopropylcyclobutanol

-
-
1199557-06-6
2-(trans-1-(4-bromophenyl)-3-cyclopropyl-3-hydroxycyclobutyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 70℃; for 38h; | 92% |
| With triethylamine In chloroform at 70℃; for 38h; | 92% |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

| Conditions | Yield |
|---|---|
| With potassium carbonate; zinc dibromide In chloroform at 0 - 20℃; for 12h; Inert atmosphere; | 92% |
| With 18-crown-6 ether; potassium carbonate; zinc dibromide In chloroform |
-
-
4603-89-8
methyl 3-amino-4,6-O-benzylidene-3-deoxy-α-D-glucopyranoside

-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
42775-84-8
methyl 4,6-O-benzylidene-3-deoxy-3-phthalimido-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With triethylamine In dimethyl sulfoxide at 23℃; for 16h; | 91% |
N-Carbethoxyphthalimide Specification
The N-Carbethoxyphthalimide, with the CAS registry number 22509-74-6, is also known as Ethyl 1,3-dioxo-1,3-dihydro-2H-isoindole-2-carboxylate. It belongs to the product categories of Biochemistry; N-Substituted Maleimides, Succinimides & Phthalimides; N-Substituted Phthalimides; Peptide Synthesis; Protection & Derivatization Reagents (for Synthesis); Protective Reagents (Peptide Synthesis); Synthetic Organic Chemistry. Its EINECS registry number is 245-048-5. This chemical's molecular formula is C11H9NO4 and molecular weight is 219.19. What's more, its IUPAC name is called Ethyl 1,3-dioxoisoindole-2-carboxylate.
Physical properties about N-Carbethoxyphthalimide are: (1)ACD/LogP: 1.472; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.47; (4)ACD/LogD (pH 7.4): 1.47; (5)ACD/BCF (pH 5.5): 7.74; (6)ACD/BCF (pH 7.4): 7.74; (7)ACD/KOC (pH 5.5): 150.62; (8)ACD/KOC (pH 7.4): 150.62; (9)#H bond acceptors: 5 ; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 63.68 Å2; (13)Index of Refraction: 1.595; (14)Molar Refractivity: 53.226 cm3; (15)Molar Volume: 156.701 cm3; (16)Polarizability: 21.101×10-24cm3; (17)Surface Tension: 59.296 dyne/cm; (18)Density: 1.399 g/cm3; (19)Flash Point: 167.815 °C; (20)Enthalpy of Vaporization: 59.884 kJ/mol; (21)Boiling Point: 353.871 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
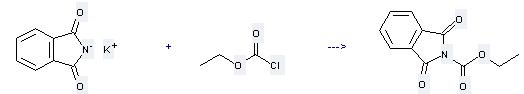
Preparation of N-Carbethoxyphthalimide: this chemical can be prepared by carbonochloridic acid ethyl ester with phthalimide; potassium salt. This reaction needs reagent 18-crown-6 and solvent toluene at temperature of 90 °C. The reaction time is 1 hour. The yield is 96 %.
Uses of N-Carbethoxyphthalimide: it is used to produce other chemicals. For example, it can react with 2-pyridin-3-yl-ethylamine to get 3-(2-phthalimidoethyl)pyridine. This reaction needs solvent ethanol at temperature of 60 °C. The reaction time is 0.5 hour. The yield is 58 %.

When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should avoid contacting with skin, eyes and wear suitable protective clothing, gloves. The gas can not be breathed. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C2c1ccccc1C(=O)N2C(=O)OCC
(2) InChI: InChI=1S/C11H9NO4/c1-2-16-11(15)12-9(13)7-5-3-4-6-8(7)10(12)14/h3-6H,2H2,1H3
(3) InChIKey: VRHAQNTWKSVEEC-UHFFFAOYSA-N
Related Products
- N-Carbethoxyphthalimide
- 22510-08-3
- 22510-10-7
- 225101-69-9
- 225104-76-7
- 225110-25-8
- 225-11-6
- 225117-51-1
- 22514-58-5
- 2251-50-5
- 22515-16-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View