-
Name
N-Isopropylacrylamide
- EINECS 218-638-5
- CAS No. 2210-25-5
- Article Data26
- CAS DataBase
- Density 0.877 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 60-63 °C(lit.)
- Formula C6H11NO
- Boiling Point 225.074 °C at 760 mmHg
- Molecular Weight 113.159
- Flash Point 119.664 °C
- Transport Information
- Appearance white to light yellow crystalline powder
- Safety 26-36/37/39
- Risk Codes 22-36-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Acrylamide,N-isopropyl- (6CI,7CI,8CI);Isopropylacrylamide;N-(1-Methylethyl)-2-propenamide;
- PSA 29.10000
- LogP 1.08790
Synthetic route

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 10H-phenothiazine; copper(II) bis(trifluoromethanesulfonate) at 140℃; for 8h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| H-ZSM-5 at 149.85℃; for 24h; Product distribution; Further Variations:; Catalysts; Addition; | 90.3% |
| With sulfuric acid; tetrabutyl-ammonium chloride; hydroquinone In water at 20 - 50℃; for 1h; Large scale; Green chemistry; | 82.3% |
| With sulfuric acid; acetic acid | |
| With sulfuric acid at 5℃; for 20h; | |
| With hydroquinone; H-ZSM-5 at 149.85℃; Product distribution; Further Variations:; Catalysts; |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 4-methoxy-phenol; lithium tert-butoxide In glycerol at 230℃; Pyrolysis; | 90% |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); 4-methoxy-phenol at 145℃; for 6h; Inert atmosphere; | 82% |
| With sulfuric acid; 4-methoxy-phenol at 180 - 220℃; under 450.045 Torr; for 6h; Reagent/catalyst; | |
| With S2O3C; styrylated-N - aminobiphenyl(DDA) at 140℃; under 52.5053 Torr; for 36h; Inert atmosphere; Ionic liquid; |

| Conditions | Yield |
|---|---|
| tetraphosphorus decasulfide; triphenyl antimony oxide In benzene at 50℃; for 6h; | 56% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 12h; | 54% |
| With aluminum oxide at 260℃; | |
| (i) 4-diethylamino-but-3-yn-2-one, THF, (ii) /BRN= 605259/; Multistep reaction; | |
| With carbon monoxide; acetone; acetylene Reagens 4:Tetracarbonylnickel; |
-
-
75-31-0
isopropylamine

-
-
503-66-2
3-hydroxypropionic acid

-
A
-
2210-25-5
N-Isopropylacrylamide

-
B
-
79-10-7
acrylic acid

| Conditions | Yield |
|---|---|
| silica gel at 250℃; Gas phase; | A 24.1% B 36.4% |


| Conditions | Yield |
|---|---|
| With aluminum oxide; phosphoric acid at 400℃; |
-
-
22813-48-5
3-chloro-propionic acid isopropylamide

-
-
121-44-8
triethylamine

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With copper at 140℃; |

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
57934-24-4
N-allyl-N-isopropyl-acrylamide

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With palladium dichloride Heating; |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With aluminum oxide; vanadia at 350℃; |

| Conditions | Yield |
|---|---|
| In dichloromethane |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 4-methoxy-phenol at 400 - 440℃; under 30.003 - 60.006 Torr; Pyrolysis; |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 10H-phenothiazine at 170 - 180℃; | 6.6 g |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2-dimethylbismuthanyl-2-methyl-propionitrile; 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 2h; Product distribution / selectivity; | 100% |
| With methyl 2-dimethylbismuthanyl-2-methylpropanoate In N,N-dimethyl-formamide at 60℃; for 6h; Product distribution / selectivity; | 99% |
| With methyl 2-dimethylbismuthanyl-2-methylpropanoate In N,N-dimethyl-formamide at 100℃; for 8h; Product distribution / selectivity; | 94% |
-
-
2210-25-5
N-Isopropylacrylamide

- poly(N-isopropylacrylamide), number-average molecular weight 2.95E4, polydispersity 1.82, tacticity m/r 81/19, reversible addition-fragmentation chain transfer polymerization; monomer(s): N-isopropylacrylamide
-
poly(N-isopropylacrylamide), number-average molecular weight 2.95E4, polydispersity 1.82, tacticity m/r 81/19, reversible addition-fragmentation chain transfer polymerization; monomer(s): N-isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); 1-Phenylethyl phenyldithioacetate; yttrium(III) trifluoromethanesulfonate In methanol; toluene at 60℃; for 4h; Kinetics; Product distribution; Further Variations:; Reagents; Solvents; | 100% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
17422-32-1
5-chloro-1H-indole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane at 50℃; | 100% |
-
-
17422-33-2
6-chloroindole

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane at 50℃; | 100% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
10601-63-5
N-(isopropyl)propionamide

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 2585.81 Torr; for 15h; Autoclave; | 99% |
| With (1+)*OTf(1-); Gantrez 149; bis[(2-diphenylphosphino)ethyl]amine hydrochloride; hydrogen at 25℃; 1.) CH3CN, 10 h; 2.) H2O; | 94% |
| With 1-methyl-pyrrolidin-2-one; hydrogen; polymer-bound Pd(0) phosphine catalyst at 20℃; | |
| With Pd*apo-ferritin; hydrogen In water-d2 at 7℃; for 1h; pH=7.5; | |
| With hydrogen In water; toluene for 2.5h; pH=3 - 7; | >= 99 %Chromat. |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2-methyl-2-methyltellanyl-propionitrile; 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 3h; | 99% |
| With ammonium persulfate; N,N'-Methylenebisacrylamide; N,N,N,N,-tetramethylethylenediamine at 4℃; for 8h; polymerization; | 82% |
| With 2,2'-azobis(isobutyronitrile) In benzene at 60℃; for 1h; |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2-dimethylstibanyl-2-methylpropionic acid ethyl ester; 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 12h; | 99% |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2-dimethylstibanyl-2-methylpropionic acid ethyl ester; 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 12h; | 99% |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With ethyl 2-methyl-2-methyl tellurium propionate; 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 6h; Product distribution / selectivity; | 99% |
| With 2,2'-azobis(isobutyronitrile); 2-dimethylstibanyl-2-methylpropionic acid ethyl ester at 60℃; for 12h; Product distribution / selectivity; Neat (no solvent); | 99% |
| With 2,2'-azobis(isobutyronitrile); 2-dimethylstibanyl-2-methylpropionic acid ethyl ester at 60℃; for 12h; Product distribution / selectivity; Neat (no solvent); | 99% |
| With 2,2'-azobis(isobutyronitrile) In methanol at 65℃; |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at 60℃; for 6h; Neat (no solvent); | 99% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 6h; | 99% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
536-74-3
phenylacetylene

-
-
1453502-79-8
N-isopropyl-5-phenyl-4-pentynamide

| Conditions | Yield |
|---|---|
| With C29H26N2O5Ru; sodium acetate In 1,4-dioxane at 100℃; for 96h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In water at 65℃; for 24h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With (1S,2S)-1,2-Cy[NC(Me)CHC(Me)N(2,6-Et2C6H3)]2YN(SiMe3)2 In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | 99% |
-
-
2210-25-5
N-Isopropylacrylamide

- poly(N-isopropylacrylamide), tacticity - meso diad 71 percent, racemo diad 29 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide
-
poly(N-isopropylacrylamide), tacticity - meso diad 71 percent, racemo diad 29 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide

| Conditions | Yield |
|---|---|
| With air; tributyl borane; yttrium(III) trifluoromethanesulfonate In methanol at -78℃; for 24h; | 98% |
-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); ytterbium(III) triflate In methanol at 60℃; | 98% |
-
-
2210-25-5
N-Isopropylacrylamide

- poly(N-isopropylacrylamide), tacticity - meso diad 84 percent, racemo diad 16 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide
-
poly(N-isopropylacrylamide), tacticity - meso diad 84 percent, racemo diad 16 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); lutetium triflate In methanol at 60℃; for 3h; | 97% |
-
-
2210-25-5
N-Isopropylacrylamide

- poly(N-isopropylacrylamide), number-average molecular weight 1.83E4, polydispersity 1.76, tacticity m/r 87/13, reversible addition-fragmentation chain transfer polymerization; monomer(s): N-isopropylacrylamide
-
poly(N-isopropylacrylamide), number-average molecular weight 1.83E4, polydispersity 1.76, tacticity m/r 87/13, reversible addition-fragmentation chain transfer polymerization; monomer(s): N-isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); 1-phenylethynyl phenyldithioacetate; ytterbium(III) triflate In methanol; toluene at 30℃; for 24h; Irradiation; | 97% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
377725-60-5
2-phenylprop-2-yl phenyldithioacetate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); yttrium(III) trifluoromethanesulfonate In methanol; toluene for 6h; Heating; | 97% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
29513-26-6
vinyl azlactone

- poly[(N-isopropyl acrylamide)-co-(vinyl azalactone)], Mw 5.1E5 Da by viscosimetry; monomer(s): N-isopropyl acrylamide, ca. 80 percent; 2-vinyl-4,4-dimethyl-2-oxazoline-5-one, ca. 20 percent
-
poly[(N-isopropyl acrylamide)-co-(vinyl azalactone)], Mw 5.1E5 Da by viscosimetry; monomer(s): N-isopropyl acrylamide, ca. 80 percent; 2-vinyl-4,4-dimethyl-2-oxazoline-5-one, ca. 20 percent

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In tert-butyl alcohol at 70℃; for 20h; | 96% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
29513-26-6
vinyl azlactone

- poly[(N-isopropyl acrylamide)-co-(vinyl azlactone)], Mw 5.1E5 Da by viscosimetry in THF at 30 deg C; monomer(s): N-isopropyl acrylamide, ca. 80 mol percent; 2-vinyl-4,4-dimethyl-2-oxazolin-5-one, ca. 20 mol percent
-
poly[(N-isopropyl acrylamide)-co-(vinyl azlactone)], Mw 5.1E5 Da by viscosimetry in THF at 30 deg C; monomer(s): N-isopropyl acrylamide, ca. 80 mol percent; 2-vinyl-4,4-dimethyl-2-oxazolin-5-one, ca. 20 mol percent

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In tert-butyl alcohol at 70℃; for 20h; | 96% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
35059-50-8
t-butyl diazoacetate

| Conditions | Yield |
|---|---|
| dmap In chlorobenzene at 20℃; for 20h; | 96% |
| dmap; CoII(3,5-DitBu-ChenPhyrin) In chlorobenzene at 20℃; for 20h; Product distribution / selectivity; Inert atmosphere; | 96% |

-
-
2210-25-5
N-Isopropylacrylamide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 60℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In N,N-dimethyl-formamide at 65℃; for 5h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| In methanol at 140℃; for 0.5h; Microwave irradiation; | 96% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1446473-49-9
diethyl 3-(isopropylamino)-3-oxopropylphosphonate

| Conditions | Yield |
|---|---|
| With C26H53N4NdSi4 at 20℃; for 0.333333h; Inert atmosphere; | 96% |
-
-
2210-25-5
N-Isopropylacrylamide

-
-
696-62-8
para-iodoanisole

-
-
132638-37-0
N-(1-methylethyl)-3-(4-methoxyphenyl)-(E)-propenamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl acetamide; water at 130℃; for 2h; Heck Reaction; | 96% |
| With sodium carbonate In N,N-dimethyl acetamide; water at 125℃; for 4h; Heck Reaction; Sealed tube; | 92% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; triethylamine In water at 100℃; for 17h; Heck Reaction; | 96% |
| With triethylamine In N,N-dimethyl-formamide at 130℃; for 5h; Heck Reaction; | 95% |
| With sodium carbonate In N,N-dimethyl acetamide; water at 130℃; for 5h; Heck Reaction; Inert atmosphere; | 94% |
| With triethylamine In N,N-dimethyl-formamide at 120℃; for 2h; Heck Reaction; | 70% |
-
-
2210-25-5
N-Isopropylacrylamide

- poly(N-isopropylacrylamide), tacticity - meso diad 67 percent, racemo diad 33 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide
-
poly(N-isopropylacrylamide), tacticity - meso diad 67 percent, racemo diad 33 percent, radical polymerization, hot water insoluble; monomer(s): N-isopropylacrylamide

| Conditions | Yield |
|---|---|
| With ytterbium(III) chloride; 2,2'-azobis(isobutyronitrile) In methanol at 60℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In tetrahydrofuran at 60℃; for 24h; | 95% |

N-Isopropylacrylamide Consensus Reports
N-Isopropylacrylamide Specification
The N-Isopropylacrylamide, with the CAS registry number 2210-25-5, is also known as 2-Propenamide, N-(1-methylethyl)-. It belongs to the product categories of Acrylamide and Methacrylamide; Acrylic Monomers; Monomers. Its EINECS registry number is 218-638-5. This chemical's molecular formula is C6H11NO and molecular weight is 113.16. What's more, its IUPAC name is called N-Propan-2-ylprop-2-enamide. It should be stored in a cool, dry and well-ventilated place.
Physical properties about N-Isopropylacrylamide are: (1)ACD/LogP: 0.278; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.28; (4)ACD/LogD (pH 7.4): 0.28; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 33.74; (8)ACD/KOC (pH 7.4): 33.74; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 29.1 Å2; (13)Index of Refraction: 1.428; (14)Molar Refractivity: 33.155 cm3; (15)Molar Volume: 128.982 cm3; (16)Polarizability: 13.144×10-24cm3; (17)Surface Tension: 25.651 dyne/cm; (18)Density: 0.877 g/cm3; (19)Flash Point: 119.664 °C; (20)Enthalpy of Vaporization: 46.159 kJ/mol; (21)Boiling Point: 225.074 °C at 760 mmHg; (22)Vapour Pressure: 0.088 mmHg at 25 °C.
Preparation of N-Isopropylacrylamide: this chemical can be prepared by isopropylamine with acrylic acid. This reaction needs catalyzer Ph3SbO/P4S10 and solvent benzene at temperature of 50 °C. The reaction time is 6 hours. The yield is 56 %.
.jpg)
Uses of N-Isopropylacrylamide: (1) it is used for producing modified latex, special coatings, adhesive; (2) it is used to produce other chemicals. For example, it can react with vinylbenzene to get N-isopropyl-trans-cinnamamide. The reaction occurs with reagent RuCHPhCl2(PCy3)(1,3-dimesityl-4,5-dihydroimidazol-2-ylidene) and solvent CH2Cl2 at temperature of 40 °C. The reaction time is 15 hours. The yield is 62 %.
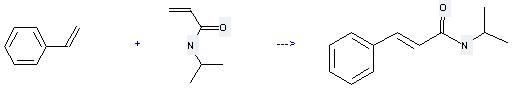
When you are dealing with this chemical, you should be very careful. This chemical may cause damage to health and it is harmful if swallowed. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: CC(C)NC(=O)C=C
(2) InChI: InChI=1S/C6H11NO/c1-4-6(8)7-5(2)3/h4-5H,1H2,2-3H3,(H,7,8)
(3) InChIKey: QNILTEGFHQSKFF-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 419mg/kg (419mg/kg) | Archives of Toxicology. Vol. 47, Pg. 179, 1981. | |
| mouse | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 6, Pg. 51, 1954. | |
| rabbit | LDLo | skin | 1580mg/kg (1580mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BLOOD: HEMORRHAGE | National Technical Information Service. Vol. OTS0545572, |
| rat | LD50 | oral | 375mg/kg (375mg/kg) | BLOOD: HEMORRHAGE PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0545572, |
Related Products
- N-Isopropylacrylamide
- 2210-28-8
- 221037-98-5
- 22104-80-9
- 22104-81-0
- 221050-88-0
- 221050-96-0
- 221054-79-1
- 2210-63-1
- 22106-33-8
- 22106-37-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View