-
Name
BOC-D-DAP-OH
- EINECS
- CAS No. 76387-70-7
- Article Data40
- CAS DataBase
- Density 1.189g/cm3
- Solubility
- Melting Point 200-203℃
- Formula C8H16 N2 O4
- Boiling Point 364.4oC at 760 mmHg
- Molecular Weight 204.226
- Flash Point 174.2oC
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Boc-D-Dap-OH
- PSA 101.65000
- LogP 1.01420
Synthetic route
-
-
7536-55-2
N-tert-butyloxycarbonylasparagine

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In water; ethyl acetate; acetonitrile at 10℃; Hofmann Rearrangement; | 90% |
| With [bis(acetoxy)iodo]benzene In water; ethyl acetate; acetonitrile at 16 - 20℃; | 88% |
| With [bis(acetoxy)iodo]benzene In water; ethyl acetate; acetonitrile at 16 - 20℃; for 4.5h; | 88% |
-
-
98541-64-1
(S)-3-(tert-butoxycarbonylamino)-oxetan-2-one

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With ammonia In tetrahydrofuran at 0℃; for 1h; | 79% |

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With pyridine; bis-[(trifluoroacetoxy)iodo]benzene 1.) aq. DMF, 15 min, 25 deg C, 2.) 4 h, 25 deg C; Yield given. Multistep reaction; |
-
-
158220-88-3
S-2-benzyloxycarbonyl-4-<(tert-butoxycarbonyl)amino>-isoxazolidin-5-one

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol for 24h; Yield given; |
-
-
71404-98-3
(R)-2-((tert-butoxycarbonyl)amino)-3-chloropropanoic acid

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93 percent / 1-ethyl-3-<3-(dimethylamino)propyl>-carbodiimide / CH2Cl2 / 15 h / Ambient temperature 2: 1.) sodium iodide, 2.) 60percent sodium hydride in mineral oil / 1.) dimethylformamide, 0 deg C, 30 min, 2.) dimethylformamide, 0 deg C, 15 min; room temperature, 90 min 3: hydrogen / 10percent Pd/C / methanol / 24 h View Scheme |
-
-
158220-90-7
1-O-(benzyloxycarbamoyl)-(N-tert-butoxycarbonyl)-β-chloro-L-alanine

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) sodium iodide, 2.) 60percent sodium hydride in mineral oil / 1.) dimethylformamide, 0 deg C, 30 min, 2.) dimethylformamide, 0 deg C, 15 min; room temperature, 90 min 2: hydrogen / 10percent Pd/C / methanol / 24 h View Scheme |
-
-
210547-97-0
(S)-3-Benzyloxyamino-2-tert-butoxycarbonylamino-propionic acid

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate / water; 1,4-dioxane / 0 - 20 °C 2: [bis(acetoxy)iodo]benzene / water; ethyl acetate; acetonitrile / 0.42 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / 1,4-dioxane; water / 16 h 2: [bis(acetoxy)iodo]benzene / water; ethyl acetate; acetonitrile View Scheme |
-
-
124730-06-9
(S)-3-Benzylamino-2-tert-butoxycarbonylamino-propionic acid

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene; palladium 10% on activated carbon In ethanol at 25℃; |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
61040-20-8
(S)-methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanoate

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether; dichloromethane at 20℃; for 2h; | 99% |
| In methanol at 20℃; for 2h; | 99% |
| In methanol; diethyl ether; dichloromethane at 20℃; for 2h; | 99% |
| In methanol; hexane; dichloromethane at 20℃; | 89% |
| In methanol |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
72071-27-3
(S)-2-tert-butoxycarbonylamino-3-(tolyl-4'-sulfonylamino)-propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium hydroxide In 1,4-dioxane; water at 0 - 20℃; for 2.5h; pH=9; Inert atmosphere; | 99% |
| With sodium carbonate In 1,4-dioxane; water at 20℃; | 91% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran; acetonitrile at 20℃; for 24h; Inert atmosphere; Darkness; | 99% |
-
-
756525-90-3
1-[(26-oxo-2,5,8,11,14,17,20,23-octaoxahexacosan-26-yl)oxy]pyrrolidine-2,5-dione

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 99% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
1493-27-2
ortho-nitrofluorobenzene

-
-
175211-37-7
(S)-2-((tert-butoxycarbonyl)amino)-3-((2-nitrophenyl)amino)-propanoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol; water for 24h; Heating / reflux; | 98% |
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 70℃; for 18h; | 83% |
| With sodium hydrogencarbonate In ethanol; water for 20h; Reflux; | 69% |
-
-
19064-24-5
2,6-difluoro-1-nitrobenzene

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 20℃; for 120h; | 97% |
| With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 20℃; for 120h; | 97% |
-
-
541-41-3
chloroformic acid ethyl ester

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0 - 20℃; for 16h; | 96% |
-
-
906564-59-8
hex-5-ynoic acid 2,5-dioxo-pyrrolidin-1-yl ester

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; | 95% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
501-53-1
benzyl chloroformate

-
-
65710-57-8
3-{[(benzyloxy)carbonyl]amino}-N-(tert-butoxycarbonyl)-L-alanine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium carbonate In tetrahydrofuran; water | 94% |
| With potassium hydroxide; potassium carbonate In tetrahydrofuran; water; toluene at -5℃; for 9h; Inert atmosphere; | 94% |
| With potassium hydroxide; potassium carbonate In tetrahydrofuran; water Inert atmosphere; | 94% |
-
-
605-65-2
5-(dimethylamino)naphth-1-ylsulfonyl chloride

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
654644-65-2
C20H27N3O6S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 12h; Inert atmosphere; | 94% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
110480-75-6
N2-BOC-N3-(3-pentynoyl)-amino-L-alanine

| Conditions | Yield |
|---|---|
| 93% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
1885-14-9
phenyl chloroformate

-
-
65710-57-8
3-{[(benzyloxy)carbonyl]amino}-N-(tert-butoxycarbonyl)-L-alanine

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 25℃; for 3h; | 93% |
-
-
35610-98-1
5-bromo-3-methyl-4-nitro-1,2-thiazole

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 25℃; for 16h; | 91% |
-
-
935676-49-6
C18H15Cl2NO4

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
935676-53-2
C26H29Cl2N3O7

| Conditions | Yield |
|---|---|
| Stage #1: C18H15Cl2NO4 With benzotriazol-1-ol; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; Stage #2: (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid With triethylamine In N,N-dimethyl-formamide for 24h; Further stages.; | 90% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
128596-03-2
11-(4-(4-methylbenzoyl)phenyl)undecanoic acid

-
-
1353054-47-3
(S)-2-(tert-butoxycarbonylamino)-3-(11-(4-(4-methylbenzoyl)phenyl)undecanamido)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 11-(4-(4-methylbenzoyl)phenyl)undecanoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane for 0.166667h; Stage #2: (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid In methanol; dichloromethane at 20℃; for 2.5h; Darkness; | 90% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
158381-70-5
N2-(tert-butoxycarbonyl),N3-(S)-2-bromosuccinamoyl,(S)-2,3-diaminopropanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Ambient temperature; | 89% |
-
-
50-00-0
formaldehyd

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 21 - 23℃; for 48h; Inert atmosphere; | 89% |
-
-
132178-37-1
2,5-dioxopyrrolidin-1-yl pent-4-ynoate

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; | 89% |
-
-
55065-02-6, 64590-80-3, 5469-45-4
N-acetamido cinnamic acid

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| Stage #1: N-acetamido cinnamic acid With benzotriazol-1-ol; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 0 - 20℃; for 3h; Stage #2: (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid With triethylamine In N,N-dimethyl-formamide for 24h; Further stages.; | 88% |
-
-
1455091-10-7
4-hydroxy-7-phenoxyisoquinoline-3-carboxylic acid methyl ester

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
1455091-19-6
2-(S)-tert-butoxycarbonylamino-3-[(4-hydroxy-7-phenoxyisoquinoline-3-carbonyl)amino]propionic acid

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 30h; Reflux; | 88% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
118715-67-6
1,3:4,6-Bis(1,4-(6-chloro-1,4-dioxahexyl)-2,3-xylylene)tetrahydro-3a,6a-diphenylimidazo<4,5-d>imidazole-2,5(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate; sodium iodide In acetonitrile for 4h; Heating; | 87% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
152120-54-2, 862686-58-6, 1143572-00-2
N,N'-bis( tert-butoxycarbonyl)-1H-pyrazole-1-carboxamidine

-
-
1248587-09-8
C19H34N4O8

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 87% |
-
-
218632-01-0
3-fluoro-4-nitrobenzonitrile

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; Inert atmosphere; | 85.4% |
-
-
23776-87-6
2,5‐dioxopyrrolidin‐1‐yl cinnamate

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
159581-09-6
(S)-2-tert-Butoxycarbonylamino-3-[(E)-(3-phenyl-acryloyl)amino]-propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol; water for 4h; | 85% |
-
-
108-24-7
acetic anhydride

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
158220-97-4
α-N-tert-butoxycarbonyl-β-acetylamino-L-alanine

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 24h; | 84% |
| With pyridine In dichloromethane at 25℃; for 16h; Inert atmosphere; |
-
-
50-00-0
formaldehyd

-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
94778-71-9
N-(tert-butoxycarbonyl)-3-(dimethylamino)-L-alanine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol; water | 84% |
| Stage #1: formaldehyd; (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid In ethanol; water at 0℃; for 0.166667h; Stage #2: With sodium cyanoborohydride at 20℃; |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
98-88-4
benzoyl chloride

-
-
538314-09-9
(S)-N2-(tert-butoxycarbonyl)-N3-benzoyl-2,3-diaminopropionic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid; benzoyl chloride With sodium hydroxide In water at 5 - 25℃; for 2h; Stage #2: With hydrogenchloride In water pH=2.5; | 83.9% |
| With sodium hydroxide In 1,4-dioxane; water at 20℃; Cooling with ice; |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
1482-97-9
(S)-2,3-diaminopropanoic acid hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane for 1h; Ambient temperature; | 83% |
-
-
76387-70-7
(S)-3-amino-2-tert-butoxycarbonylaminopropionic acid

-
-
147299-36-3
6-<4-(4-n-hexylbenzoyl)phenyl>hexanoic acid

-
-
1353054-42-8
(S)-2-(tert-butoxycarbonylamino)-3-(6-(4-(4-hexylbenzoyl)phenyl)hexanamido)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 6-<4-(4-n-hexylbenzoyl)phenyl>hexanoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane for 0.166667h; Stage #2: (S)-3-amino-2-tert-butoxycarbonylaminopropionic acid In methanol; dichloromethane at 20℃; for 12h; Darkness; | 83% |
N-alpha-Boc-D-2,3-diaminopropionic acid Chemical Properties
Product Name: N-alpha-Boc-D-2,3-diaminopropionic acid (CAS NO.76387-70-7)
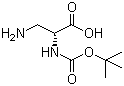
Molecular Formula: C8H16N2O4
Molecular Weight: 204.22g/mol
Mol File: 76387-70-7.mol
Boiling point: 364.4 °C at 760 mmHg
Storage Temperature: Store at RT.
Flash Point: 174.2 °C
Density: 1.189 g/cm3
Surface Tension: 45.7 dyne/cm
Enthalpy of Vaporization: 67.07 kJ/mol
Vapour Pressure: 2.64E-06 mmHg at 25°C
Related Products
- N-alpha-Boc-D-2,3-diaminopropionic acid
- 76387-79-6
- 76391-97-4
- 76393-18-5
- 763932-69-0
- 76397-72-3
- 764-01-2
- 76408-00-9
- 76408-62-3
- 76408-68-9
- 76408-71-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View