-
Name
Anilinoacetic acid
- EINECS 203-070-2
- CAS No. 103-01-5
- Article Data101
- CAS DataBase
- Density 1.259 g/cm3
- Solubility moderately soluble in water
- Melting Point 121-127 °C
- Formula C8H9NO2
- Boiling Point 359 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 170.9 °C
- Transport Information
- Appearance ochre to yellow-brown fine powder
- Safety 22-24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms H-DL-Phg-OH;TR-NPG;(Phenylamino)aceticacid;Acetic acid, (phenylamino)-;Anilinoacetic acid;N-(Phenylamino)aceticacid;N-Phenylglycine;NSC 83567;
- PSA 49.33000
- LogP 1.25610
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: ethyl bromoacetate; aniline With silica gel at 20℃; for 0.116667h; microwave irradiation; Stage #2: With sodium hydroxide In ethanol for 0.166667h; Heating; | 93% |
| Stage #1: ethyl bromoacetate; aniline With potassium carbonate for 1.5h; Milling; Stage #2: With potassium hydroxide for 1.5h; Milling; | 91% |
| Stage #1: ethyl bromoacetate; aniline Stage #2: With sodium hydroxide |

| Conditions | Yield |
|---|---|
| 90% |
-
-
65171-67-7
tert-butyl 2-(phenylamino)acetate

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane for 3h; | 90% |
| In water; isopropyl alcohol at 200℃; for 0.25h; Solvent; Temperature; Flow reactor; | 77% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; 2-(N,N-dimethylamino)ethanol In water at 75 - 80℃; for 72h; | 87% |
| With copper(l) iodide; 2-(2-methyl-1-oxopropyl)cyclohexanone; caesium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; | 82% |
| With copper(I) oxide; caesium carbonate at 100℃; for 0.666667h; Sealed tube; Microwave irradiation; | 10% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 2-(2-methyl-1-oxopropyl)cyclohexanone; potassium carbonate In water at 90℃; for 0.833333h; Microwave irradiation; Green chemistry; | 81% |
| With pectin-stabilized copper nanoparticles In dimethyl sulfoxide at 110℃; for 2h; | 71% |
| With potassium phosphate; copper(l) iodide; 2-(N,N-dimethylamino)ethanol In water at 90℃; | 62% |
| With copper(l) iodide; potassium carbonate In N,N-dimethyl acetamide at 90℃; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 0.5h; Heating; | 73% |
| With sodium hydroxide for 0.5h; Heating; | 70% |
| Stage #1: N-phenylglycine ethyl ester With sodium hydroxide In water for 0.5h; Reflux; Stage #2: With hydrogenchloride In water pH=2; |
-
-
178695-68-6, 181121-53-9
1-phenyl-4-oximinoimidazolidine

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride Hydrolysis; | 60% |
-
-
591-50-4
iodobenzene

-
-
556-50-3
glycylglycine

-
A
-
852380-00-8
N-(N-phenyl-glycyl)-glycine

-
B
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; 2-(N,N-dimethylamino)ethanol In water at 70℃; for 16h; | A 40% B 27% |


| Conditions | Yield |
|---|---|
| With sulfuric acid Electrolysis.elektrolytische Reduktion; |

| Conditions | Yield |
|---|---|
| With potassium cyanide; ethanol |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With calcium hydroxide; alkali carbonate; water | |
| With water In aq. phosphate buffer at 37℃; pH=7; Green chemistry; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With water; zinc | |
| With sulfuric acid Electrolysis.elektrolytische Reduktion; |

| Conditions | Yield |
|---|---|
| With alkali |
-
-
39126-37-9
anilino-malonic acid

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With water |
-
-
861321-16-6
N-anilinomethyl formanilide

-
-
151-50-8
potassium cyanide

-
A
-
21969-70-0
2-anilinoacetamide

-
C
-
103-01-5
N-Phenylglycine

-
D
-
62-53-3
aniline


| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| man verseift den entstehenden Anilinoessigsaeure-aethylester durch Kochen mit alkoh.Kalilauge; | |
| With water bei Gegenwart eines salzsaeurebindenen Mittels (anorganischer Basen oder basich wirkender Salze); man verseift den entstandenen Anilinoessigsaeure-aethylester durch Kochen mit alkoh.Kalilauge; | |
| Anilinoessigsaeure-aethylester entsteht; | |
| With water bei Gegenwart eines salzsaeurebindenen Mittels (anorganischer Basen oder basich wirkender Salze); Anilinoessigsaeure-aethylester entsteht; |

| Conditions | Yield |
|---|---|
| With iron |

| Conditions | Yield |
|---|---|
| With water; sodium acetate | |
| With iron(II) oxide; sodium chloride | |
| With sodium chloride bei Gegenwart von Carbonaten der Schwermetalle; |
-
-
79-11-8
chloroacetic acid

-
-
62-53-3
aniline

-
A
-
1137-73-1
N,N-bis(carboxymethyl)aniline

-
B
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With water; sodium acetate ueber mehrere Stufen; |


| Conditions | Yield |
|---|---|
| With water man trennt die Reaktionsprodukte durch wiederholte fraktionierte Krystallisation aus Wasser, wobei sich das Anilinsalz der Anilin-N.N-diessigsaeure zuletzt abscheidet; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-phenylglycine potassium salt |


| Conditions | Yield |
|---|---|
| With alkali cyanide |

| Conditions | Yield |
|---|---|
| With calcium hydroxide; water at 180℃; |

| Conditions | Yield |
|---|---|
| man verseift den entstandenen Anilinoessigsaeure-isoamylester mit konz.waessr.Natronlauge; |

| Conditions | Yield |
|---|---|
| In water for 0.5h; |

| Conditions | Yield |
|---|---|
| Alkylation; |
-
-
20635-51-2
1,4-diphenylpiperazine-2,5-dione

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| In toluene for 0.5h; Heating; | 100% |
| In benzene at 80℃; for 1h; | 96% |
| In toluene Heating; | 96% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
103-01-5
N-Phenylglycine

-
-
150806-61-4
N-t-butoxycarbonyl L-phenylglycine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water | 100% |
| With triethylamine In water; acetone at 20℃; for 19h; | 75% |
| With sodium hydroxide In 1,4-dioxane-water; citric acid | |
| With triethylamine In water; acetone at 20℃; |
-
-
103-01-5
N-Phenylglycine

-
-
78733-46-7
methyl (+/-)-phenylglycinate hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In methanol | 100% |

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 24h; Sealed tube; Irradiation; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 5h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; Irradiation; Inert atmosphere; diastereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; lithium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 20℃; | 98% |
| With sulfuric acid for 1h; Heating; | 85% |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; Green chemistry; | 98% |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
103-01-5
N-Phenylglycine

-
-
1181454-77-2
1-(4-fluorophenyl)-2-(phenylamino)ethan-1-ol

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: C17H17N3O2; N-Phenylglycine With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 15h; Sealed tube; Irradiation; Inert atmosphere; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; for 0.0833333h; Sealed tube; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With nickel(II) triflate; P(p-CH3OC6H4)3; Ir(dFCF3(ppy))2(4,4’-ditert-butyl-2,2’-bipyrididyl)PF6; caesium carbonate In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With lithium hexafluorophosphate; 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile; C31H21F6O4P In tetrahydrofuran at -35℃; Inert atmosphere; Irradiation; enantioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 5h; Sealed tube; Irradiation; Inert atmosphere; diastereoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; lithium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 97% |
-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With sodium In butan-1-ol | 96.3% |

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; Green chemistry; | 96% |
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water for 4h; Catalytic behavior; Solvent; Schlenk technique; Sealed tube; Irradiation; | 96.2% |

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 9h; Sealed tube; Irradiation; Inert atmosphere; diastereoselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 5h; Sealed tube; Irradiation; Inert atmosphere; diastereoselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 8h; Sealed tube; Irradiation; Inert atmosphere; diastereoselective reaction; | 96% |
-
-
103-01-5
N-Phenylglycine

-
-
508191-77-3
3-phenylsydnone

| Conditions | Yield |
|---|---|
| With N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide; acetic anhydride; sodium nitrite In dichloromethane at 0 - 5℃; for 5h; | 95% |
| Stage #1: N-Phenylglycine With sodium nitrite Milling; Stage #2: With trifluoroacetic anhydride Milling; | 95% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; acetic anhydride; sodium nitrite In dichloromethane at 0 - 5℃; for 10h; | 94% |

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; triethylamine In methanol; dichloromethane; chloroform; acetonitrile | 95% |

-
-
103-01-5
N-Phenylglycine

| Conditions | Yield |
|---|---|
| In acetonitrile for 5h; Reflux; Molecular sieve; | 95% |

| Conditions | Yield |
|---|---|
| With nickel(II) triflate; P(p-CH3OC6H4)3; Ir(dFCF3(ppy))2(4,4’-ditert-butyl-2,2’-bipyrididyl)PF6; caesium carbonate In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; Inert atmosphere; | 95% |
-
-
66107-32-2
4-cyanophenyl trifluoromethanesulfonate

-
-
103-01-5
N-Phenylglycine

-
-
37812-49-0
4-phenylaminomethyl-benzonitrile

| Conditions | Yield |
|---|---|
| With nickel(II) triflate; P(p-CH3OC6H4)3; Ir(dFCF3(ppy))2(4,4’-ditert-butyl-2,2’-bipyrididyl)PF6; caesium carbonate In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; Inert atmosphere; | 95% |
-
-
17763-71-2
4-carbomethoxyphenyl triflate

-
-
103-01-5
N-Phenylglycine

-
-
39126-16-4
N-[4-(methyloxycarbonyl)benzyl]aniline

| Conditions | Yield |
|---|---|
| With nickel(II) triflate; P(p-CH3OC6H4)3; Ir(dFCF3(ppy))2(4,4’-ditert-butyl-2,2’-bipyrididyl)PF6; caesium carbonate In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With lithium hexafluorophosphate; 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile; C31H21F6O4P In tetrahydrofuran at -35℃; Inert atmosphere; Irradiation; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate In water at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; Green chemistry; | 95% |

-
-
103-01-5
N-Phenylglycine

-
-
7148-74-5
2-Bromopropionyl chloride

-
B
-
861570-46-9
N-(2-bromo-propionyl)-N-phenyl-glycine

| Conditions | Yield |
|---|---|
| A 94.8% B n/a |

-
-
909114-66-5
9-fluorenylmethyl 4,6-dimethoxy-1,3,5-triazinyl carbonate

-
-
103-01-5
N-Phenylglycine

-
-
909114-68-7
N-{[(9H-fluoren-9-yl)meth-1-yl-oxy]carbonyl}-N-phenylglycine

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetonitrile at 20℃; for 1.08333h; | 94% |
N-phenylglycine Chemical Properties
N-phenylglycine(103-01-5) has a melting point of 121-123 °C(lit.).
The chemical synonyms of N-phenylglycine(103-01-5) are AURORA KA-3653;ANILINOACETIC ACID;H-PHENYLGLY-OH;AKOS B003332;RARECHEM AL BO 1139;N-PHENYLGLYCIN;N-PHENYLGLYCINE;N-PHENYL-GLY-OH
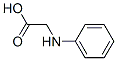
The molecular structure of N-phenylglycine(103-01-5):
N-phenylglycine Toxicity Data With Reference
LD50/LC50: RTECS: Not available.
Carcinogenicity: N-PHENYLGLYCINE, 93%(TLC) - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
Other: The toxicological properties have not been fully investigated.
N-phenylglycine Safety Profile
WGK Germany 3
HS Code 29224995
N-phenylglycine Specification
Conditions to Avoid: Incompatible materials.
Incompatibilities with Other Materials Strong oxidizing agents.
Hazardous Decomposition Products Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization Has not been reported.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View