-
Name
Nitroterephthalic acid
- EINECS 210-217-4
- CAS No. 610-29-7
- Article Data28
- CAS DataBase
- Density 1.671 g/cm3
- Solubility
- Melting Point 270-272 °C(lit.)
- Formula C8H5NO6
- Boiling Point 454.781 °C at 760 mmHg
- Molecular Weight 211.131
- Flash Point 205.463 °C
- Transport Information
- Appearance white to off-white powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Terephthalicacid, nitro- (6CI,7CI,8CI);2-Nitro-p-phthalic acid;2-Nitrobenzene-1,4-dicarboxylic acid;2-Nitroterephthalic acid;NSC 48124;Terephthalic acid, nitro-;
- PSA 120.42000
- LogP 1.51440
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 60℃; for 5h; | 98% |

| Conditions | Yield |
|---|---|
| With fuming sulphuric acid; nitric acid at 25 - 90℃; for 4h; | 85% |
| With sulfuric acid; nitric acid at 5 - 60℃; for 1h; | 68.6% |
| With sulfuric acid; nitric acid |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; permanganate(VII) ion |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium hexacyanoferrate(III) |
-
-
84540-59-0
4-methyl-3-nitrobenzyl chloride

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion |
-
-
85062-97-1
4-methyl-2-nitro-benzyl chloride

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| With air; nitric acid |

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| bei der Oxydation; |
-
-
778-94-9
2-nitro-4-trifluoromethyl-benzonitrile

-
-
7664-93-9
sulfuric acid

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| at 190℃; |
-
-
100-21-0
terephthalic acid

-
-
7697-37-2
nitric acid

-
-
7783-05-3
disulfuric acid

-
-
610-29-7
2-nitroterephthalic acid


| Conditions | Yield |
|---|---|
| at 180℃; |
-
-
84540-59-0
4-methyl-3-nitrobenzyl chloride

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid / Hydrolysis 2: permanganate; NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: durch Nitrierung 2: sulfuric acid / Hydrolysis 3: permanganate; NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite In water at 0 - 5℃; for 1h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / 0.67 h / 70 °C 2: nitric acid; sulfuric acid / 1 h / 5 - 60 °C View Scheme |
-
-
610-29-7
2-nitroterephthalic acid

-
-
10312-55-7
3-aminoterephthalic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; palladium on activated charcoal In water under 2585.7 Torr; for 12h; | 100% |
| With palladium 10% on activated carbon; ammonium formate; silica gel In methanol for 1.5h; Milling; | 99% |
| With hydrogen In ethanol at 20℃; under 760.051 Torr; for 4h; | 92% |
-
-
64479-78-3
N-(pyrid-4-yl)isonicotinamide

-
-
610-29-7
2-nitroterephthalic acid

-
-
5893-61-8, 14690-98-3, 15305-21-2, 16842-73-2, 22992-79-6, 34729-06-1, 133386-02-4, 133386-04-6
copper(II) formate tetrahydrate

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitroterephthalic acid; copper(II) formate tetrahydrate In methanol at 39.84℃; for 24h; Inert atmosphere; Stage #2: N-(pyrid-4-yl)isonicotinamide In methanol at 39.84℃; for 24h; | 98% |

-
-
610-29-7
2-nitroterephthalic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 23℃; for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid; silver carbonate In dimethyl sulfoxide at 120℃; for 16h; regioselective reaction; | 93% |
| With copper(l) iodide; triethylamine In dimethyl sulfoxide at 120℃; under 760.051 Torr; for 20h; Inert atmosphere; Schlenk technique; regioselective reaction; | 41% |
-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran at 0 - 40℃; for 19h; | 92% |
| With dimethylsulfide borane complex In tetrahydrofuran at 0 - 40℃; for 18h; Inert atmosphere; | 92% |
| With borane-THF In tetrahydrofuran at 40℃; Inert atmosphere; Cooling with ice; | 90% |
| With diborane |

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran at 80℃; for 24h; Sealed tube; | 92% |

-
-
610-29-7
2-nitroterephthalic acid

-
-
67-68-5
dimethyl sulfoxide

-
-
1135-32-6
trans-1,2-bis(4-pyridyl)ethylene

| Conditions | Yield |
|---|---|
| With air In N,N-dimethyl-formamide at 120℃; for 24h; Autoclave; | 90% |


-
-
610-29-7
2-nitroterephthalic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| at 80℃; for 36h; | 90% |

-
-
66-71-7
1,10-Phenanthroline

-
-
610-29-7
2-nitroterephthalic acid

-
-
7732-18-5
water

-
-
832672-75-0, 639029-41-7
([Cu(2-nitro-1,4-benzenedicarboxylate dianion)(1,10-phenanthroline)](H2O))n

| Conditions | Yield |
|---|---|
| In water High Pressure; mixt. of Cu-compound, organic ligands and water sealed in a stainless-steel reactor with Teflon liner, heated at 150°C for 3 h, cooled; elem. anal.; | 87% |


| Conditions | Yield |
|---|---|
| at 100℃; for 83.3333h; Heating; | 87% |


| Conditions | Yield |
|---|---|
| In ethanol; N,N-dimethyl-formamide at 89.84℃; for 24h; Inert atmosphere; | 87% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 110℃; for 92h; | 85% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 110℃; for 92h; | 85% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 110℃; for 92h; | 85% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide mixt. of stoich. amt. of CuCl2, nitro compd. and phen were dissolved in DMF; crystn. at room temp for 1 mo, elem. anal.; | 83% |


-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| With Et3N In water High Pressure; Cu-salt:acid:H2O molar ratio 1:1:4163, Et3N was added, heating in stainless-steel reactor with teflon liner at 170°C for 2 d; cooling, crystals were collected by filtration, elem. anal.; | 83% |
-
-
610-29-7
2-nitroterephthalic acid

-
-
66881-27-4
2,2,2-Trinitroethyl hydrogen sulfate

| Conditions | Yield |
|---|---|
| at 100℃; for 15h; | 78% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitroterephthalic acid With propynoic acid ethyl ester In 1,4-dioxane at 80℃; Stage #2: N,N'-dimethylbenzylamine In 1,4-dioxane at 80℃; for 12h; | 77% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 80℃; for 5h; | 76% |
| With thionyl chloride Heating; | 75% |
| With sulfuric acid Reflux; | 27% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dimethyl-4H-1,2,4-triazol-4-amine; 2-nitroterephthalic acid; silver nitrate With air In water for 0.5h; Stage #2: In water at 100℃; for 72h; Autoclave; | 72.24% |


-
-
64479-78-3
N-(pyrid-4-yl)isonicotinamide

-
-
610-29-7
2-nitroterephthalic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 119.84℃; for 24h; Inert atmosphere; | 72% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 110℃; for 92h; | 72% |


| Conditions | Yield |
|---|---|
| With sulfuric acid for 1.25h; Heating; | 71% |
| With sulfuric acid for 1h; Heating; | 55% |
| With hydrogenchloride | |
| With sulfuric acid for 1h; Heating; | 14.7 g |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; | 71% |

-
-
610-29-7
2-nitroterephthalic acid

-
-
10026-11-6
zirconium(IV) chloride

| Conditions | Yield |
|---|---|
| In acetic acid; N,N-dimethyl-formamide at 240℃; for 0.0166667h; Microwave irradiation; | 70.3% |

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; under 760.051 Torr; for 3.5h; Sonication; | 69% |

-
-
610-29-7
2-nitroterephthalic acid

-
-
14667-55-1
2,3,5-trimethylpyrazine

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitroterephthalic acid; 2,3,5-trimethylpyrazine; silver nitrate With air In water for 0.5h; Stage #2: In water at 100℃; for 72h; Autoclave; | 68.72% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide; water at 100℃; for 72h; Sealed tube; | 65% |

Nitroterephthalic acid Specification
The Nitroterephthalic acid, with the CAS registry number 610-29-7, is also known as Terephthalic acid, nitro-. It belongs to the product categories of Phthalic Acids, Esters and Derivatives; Derivatives of phthalic acid; C8; Carbonyl Compounds; Carboxylic Acids. Its EINECS number is 210-217-4. This chemical's molecular formula is C8H5NO6 and molecular weight is 211.13. What's more, its systematic name is 2-Nitroterephthalic acid. It should be sealed and stored.
Physical properties of Nitroterephthalic acid are: (1)ACD/LogP: 2.007; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.10; (4)ACD/LogD (pH 7.4): -2.14; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 7; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 120.42 Å2; (13)Index of Refraction: 1.66; (14)Molar Refractivity: 46.658 cm3; (15)Molar Volume: 126.334 cm3; (16)Polarizability: 18.497×10-24cm3; (17)Surface Tension: 88.07 dyne/cm; (18)Density: 1.671 g/cm3; (19)Flash Point: 205.463 °C; (20)Enthalpy of Vaporization: 75.282 kJ/mol; (21)Boiling Point: 454.781 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
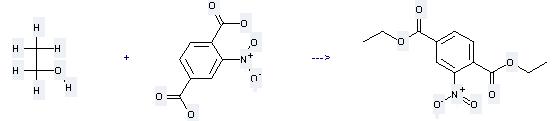
Uses of Nitroterephthalic acid: it can be used to produce nitro-terephthalic acid diethyl ester by heating. It will need reagent H2SO4 with the reaction time of 24 hours. The yield is about 59%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: O=[N+]([O-])c1cc(ccc1C(=O)O)C(=O)O
(2)Std. InChI: InChI=1S/C8H5NO6/c10-7(11)4-1-2-5(8(12)13)6(3-4)9(14)15/h1-3H,(H,10,11)(H,12,13)
(3)Std. InChIKey: QUMITRDILMWWBC-UHFFFAOYSA-N
Related Products
- Nitroterephthalic acid
- 610-30-0
- 610302-03-9
- 610309-89-2
- 61032-42-6
- 610-34-4
- 61034-86-4
- 610-35-5
- 61035-87-8
- 610-36-6
- 61036-62-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View