-
Name
DIMETHYL TRIMETHYLSILYL PHOSPHITE
- EINECS 252-908-3
- CAS No. 36198-87-5
- Article Data12
- CAS DataBase
- Density 0.954 g/mL at 25 °C(lit.)
- Solubility
- Melting Point
- Formula C5H15O3PSi
- Boiling Point 146.6 °C at 760 mmHg
- Molecular Weight 182.232
- Flash Point 38.9 °C
- Transport Information UN 2920 8/PG 2
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 10-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Dimethyltrimethylsilyl phosphite;Trimethylsilyl dimethyl phosphite;Dimethoxy[(trimethylsilyl)oxy]phosphine;
- PSA 41.28000
- LogP 2.35760
Synthetic route
-
-
868-85-9
Dimethyl phosphite

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| at 90℃; for 2h; | 89% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
868-85-9
Dimethyl phosphite

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| Stage #1: Dimethyl phosphite With triethylamine In dichloromethane at 0℃; Inert atmosphere; Stage #2: chloro-trimethyl-silane In dichloromethane at 0℃; for 0.5h; Inert atmosphere; | 75% |
| With triethylamine In dichloromethane at 0℃; Inert atmosphere; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
868-85-9
Dimethyl phosphite

-
A
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; | A 75% B n/a |

-
-
75-77-4
chloro-trimethyl-silane

-
-
96-36-6, 868-85-9
methyl phosphite

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane In hexane for 1h; Heating; | 67% |
| With triethylamine In benzene | |
| With sodium 1.) ether, 2.) ether, RT, 1.5 h; Yield given. Multistep reaction; | |
| With triethylamine In dichloromethane at 0℃; | |
| With triethylamine In dichloromethane at 0℃; for 1h; |
-
-
18135-34-7
O,O-dimethyl dithiophosphoric acid S-trimethylsilyl ester

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
2953-29-9
dimethyl S-methyl phosphorodithioate

-
B
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| In diethyl ether Yield given; |

-
-
2857-97-8
trimethylsilyl bromide

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| In dichloromethane for 1.25h; |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
121-44-8
triethylamine

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite With borane-THF In tetrahydrofuran Stage #2: triethylamine In methanol | 100% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
98-86-2
acetophenone

-
-
36190-36-0
(1-phenyl-1-trimethylsilanyloxy-ethyl)-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With C4H9O(1-)*C13H27KO6(1+) In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With diethyl ether; lithium perchlorate at 20℃; for 0.25h; | 99% |

-
-
1468-83-3
1-(thiophen-3-yl)-ethanone

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
1172141-67-1
(1-thiophen-3-yl-1-trimethylsilanyloxy-ethyl)-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With C4H9O(1-)*C13H27KO6(1+) In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; | 99% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
93-55-0
1-phenyl-propan-1-one

-
-
1172141-66-0
(1-phenyl-1-trimethylsilanyloxy-propyl)-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With C4H9O(1-)*C13H27KO6(1+) In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; | 99% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
13707-47-6
2-furaldehyde tosylimine

-
-
1154063-26-9
dimethyl (furan-2-yl)-(4-methylphenylsulfonamido)methylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; 2-furaldehyde tosylimine In tetrahydrofuran at 0 - 20℃; for 5h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 99% |
| With iodine In dichloromethane at 0 - 20℃; Inert atmosphere; | 86% |
| With Amberlyst-15 In dichloromethane at 0℃; for 3h; Inert atmosphere; | 86% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
198012-02-1
4-methyl-N-(4-trifluoromethylbenzylidene)-benzenesulfonamide

-
-
1396319-24-6
dimethyl (((4-methylphenyl)sulfonamido)(4-(trifluoromethyl)phenyl)methyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; 4-methyl-N-(4-trifluoromethylbenzylidene)-benzenesulfonamide In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 99% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
81323-59-3
tert-butyl (2S)-2-[(tert-butoxycarbonyl)amino]-4-oxobutanoate

-
-
130568-04-6
t-butyl (2S,4RS)-2-(t-butoxycarbonylamino)-4-(dimethylphosphono)-4-hydroxybutanoate

| Conditions | Yield |
|---|---|
| In acetonitrile for 1.5h; Heating; | 98% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
13707-46-5
N-tosyl-4-nitrobenzaldimine

-
-
1154063-22-5
dimethyl (((4-methylphenyl)sulfonamido)(4-nitrophenyl)methyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; N-tosyl-4-nitrobenzaldimine In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 98% |
| With iodine In dichloromethane at 0 - 20℃; Inert atmosphere; | 91% |
| With Amberlyst-15 In dichloromethane at 0℃; for 3.5h; Inert atmosphere; | 86% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
73845-02-0
4-methyl-N-(3-nitrobenzylidene)benzenesulfonamide

-
-
1373284-91-3
dimethyl (4-methylphenylsulfonamido)(3-nitrophenyl)methylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; 4-methyl-N-(3-nitrobenzylidene)benzenesulfonamide In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 98% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
100-65-2
N-Phenylhydroxylamine

-
-
78-84-2
isobutyraldehyde

| Conditions | Yield |
|---|---|
| With diethyl ether; lithium perchlorate at 20℃; for 0.25h; | 97% |


| Conditions | Yield |
|---|---|
| Stage #1: propionaldehyde; N,N-Dimethylhydrazine With lithium perchlorate In diethyl ether at 20℃; for 0.0833333h; Stage #2: dimethyl trimethylsilyl phosphite In diethyl ether at 20℃; for 0.25h; | 97% |

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
66-99-9
β-naphthaldehyde

| Conditions | Yield |
|---|---|
| With 3,3’-bis[3,5-bis(trifluoromethyl)phenyl]-1,1’-binaphthalene-2,2’-sulfonimide In diethyl ether at -78℃; for 96h; Abramov Phosphorylation; enantioselective reaction; | 97% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane for 0.25h; Inert atmosphere; Reflux; regioselective reaction; | 97% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
2720-41-4
cyclohexanethione

-
-
79967-05-8
(1-Trimethylsilanylsulfanyl-cyclohexyl)-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| at 5 - 20℃; | 96% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
57-14-7
1,1-dimethylhydrazine

-
-
2043-61-0
cyclohexanecarbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-Dimethylhydrazine; cyclohexanecarbaldehyde With lithium perchlorate In diethyl ether at 20℃; for 0.0833333h; Stage #2: dimethyl trimethylsilyl phosphite In diethyl ether at 20℃; for 0.25h; | 96% |

-
-
66-71-7
1,10-Phenanthroline

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane at 45℃; Inert atmosphere; Microwave irradiation; Sealed tube; | 96% |
-
-
18135-15-4
thiophosphoric acid O,O'-dimethyl ester-O''-trimethylsilanyl ester

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
71861-22-8
tetramethyl symmetrical monothiopyrophosphate

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In dichloromethane -10 deg C then -40 deg C to R.T.; | 95% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
71861-22-8
tetramethyl symmetrical monothiopyrophosphate

| Conditions | Yield |
|---|---|
| With sulfur dichloride 1.) from -50 deg C to r.t.; 2.) r.t., 15 min; | 95% |
| With sulfur dichloride In dichloromethane at -50℃; |

| Conditions | Yield |
|---|---|
| With diethyl ether; lithium perchlorate at 20℃; for 0.25h; | 95% |

-
-
14371-10-9
(E)-3-phenylpropenal

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
100-65-2
N-Phenylhydroxylamine

| Conditions | Yield |
|---|---|
| With diethyl ether; lithium perchlorate at 20℃; for 0.25h; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: butyraldehyde; N,N-Dimethylhydrazine With lithium perchlorate In diethyl ether at 20℃; for 0.0833333h; Stage #2: dimethyl trimethylsilyl phosphite In diethyl ether at 20℃; for 0.25h; | 95% |

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
75159-10-3
N-(4-methylbenzylidene)-p-toluenesulfonamide

-
-
1154063-25-8
dimethyl (4-methylphenyl)(4-methylphenylsulfonamido)methylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; N-(4-methylbenzylidene)-p-toluenesulfonamide In tetrahydrofuran at 0 - 20℃; for 5h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 95% |
| With iodine In dichloromethane at 0 - 20℃; Inert atmosphere; | 92% |
| With Amberlyst-15 In dichloromethane at 0℃; for 2.5h; Inert atmosphere; | 91% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
63160-16-7
4-nitro-N-(phenylmethylene)benzenesulfonamide

-
-
1373284-94-6
dimethyl [{[(4-nitrophenyl)sulfonyl]amino}(phenyl)methyl]phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; 4-nitro-N-(phenylmethylene)benzenesulfonamide In tetrahydrofuran at 0 - 20℃; for 3h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 95% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
175355-34-7
N-(cyclohexylmethylene)-4-methylbenzenesulfonamide

-
-
1373284-93-5
dimethyl cyclohexyl(4-methylphenylsulfonamido)methylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; N-(cyclohexylmethylene)-4-methylbenzenesulfonamide In tetrahydrofuran at 0 - 20℃; for 5h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: pentanal; N,N-Dimethylhydrazine With lithium perchlorate In diethyl ether at 20℃; for 0.0833333h; Stage #2: dimethyl trimethylsilyl phosphite In diethyl ether at 20℃; for 0.25h; | 94% |


| Conditions | Yield |
|---|---|
| Stage #1: N,N-Dimethylhydrazine; isobutyraldehyde With lithium perchlorate In diethyl ether at 20℃; for 0.0833333h; Stage #2: dimethyl trimethylsilyl phosphite In diethyl ether at 20℃; for 0.25h; | 94% |


-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
151426-18-5
tetramethyl 4-(4-methylthiophenyl)thio-1-trimethylsiloxybutylidene-1,1-diphosphonate

| Conditions | Yield |
|---|---|
| With phosphorous acid trimethyl ester In tetrahydrofuran | 94% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
150884-50-7
benzaldehyde N-boc imine

-
-
71864-40-9
carbamic acid N-[(dimethoxyphosphinyl)phenylmethyl] 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl trimethylsilyl phosphite; benzaldehyde N-boc imine In tetrahydrofuran at 0 - 20℃; for 5h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 94% |
| With C4H9O(1-)*C13H27KO6(1+) In tetrahydrofuran at -40℃; for 3h; Inert atmosphere; | 83% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
51608-60-7
N-(benzylidene)-p-methylbenzenesulfonamide

-
-
59191-36-5
dimethyl (((4-methylphenyl)sulfonamido)(phenyl)methyl)phosphonate

| Conditions | Yield |
|---|---|
| With Amberlyst-15 In dichloromethane at 0℃; for 2h; Inert atmosphere; | 94% |
| With iodine In dichloromethane at 0 - 20℃; Inert atmosphere; | 93% |
| Stage #1: dimethyl trimethylsilyl phosphite; N-(benzylidene)-p-methylbenzenesulfonamide In tetrahydrofuran at 0 - 20℃; for 24h; Inert atmosphere; Stage #2: With water In tetrahydrofuran for 0.5h; Inert atmosphere; | 93% |
Phosphorous acid,dimethyl trimethylsilyl ester Specification
The Phosphorous acid,dimethyl trimethylsilyl ester, with CAS registry number of 36198-87-5, is also known as Trimethylsilyl dimethyl phosphite. It belongs to categories of Organic Building Blocks; Organic Phosphates/Phosphites; Phosphorus Compounds. Its IUPAC name is didecyl phenyl phosphite.
Physical properties about this chemical are: (1) ACD/LogP: 3.51; (2) # of Rule of 5 Violations: 0; (3) ACD/LogD (pH 5.5): 3.51; (4) ACD/LogD (pH 7.4): 3.51; (5) ACD/BCF (pH 5.5): 272.26; (6) ACD/BCF (pH 7.4): 272.26; (7) ACD/KOC (pH 5.5): 1925.59; (8) ACD/KOC (pH 7.4): 1925.59; (9) # H bond acceptors: 3; (10) # H bond donors: 0; (11) # Freely Rotating Bonds: 4; (12) Polar Surface Area: 41.28 Å2; (13) Flash Point: 38.9 °C; (14) Enthalpy of Vaporization: 36.78 kJ/mol; (15) Boiling Point: 146.6 °C at 760 mmHg; (16) Vapour Pressure: 5.82 mmHg at 25°C.
Preparation of Phosphorous acid,dimethyl trimethylsilyl ester: it is prepared by reaction of chloro-trimethyl-silane with phosphonic acid dimethyl ester. The reaction needs reagent hexamethyldisilazane and solvent hexane as well as other condition of heating for 1 hour. The yield is about 67%.
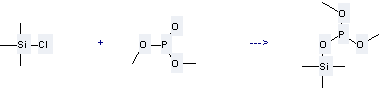
Uses of Phosphorous acid,dimethyl trimethylsilyl ester: It is used to produce other chemicals. For example, it can produce Dimethyl-1-oxononanphosphonat. It reacts with nonanoyl chloride at the temperature of 50 °C for 30 min. The yield is 69.8%.
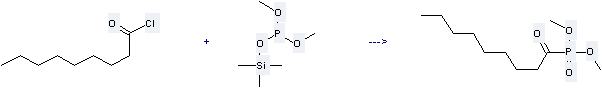
When you are using this chemical, please be cautious about it as the following:
As a chemical, it is corrosive, and may destroy living tissue on contact. Meanwhile, it is flammable and may causes burns. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O(P(OC)O[Si](C)(C)C
(2)InChI: InChI=1/C5H15O3PSi/c1-6-9(7-2)8-10(3,4)5/h1-5H3
(3)InChIKey: HWMXPTIFAGBDIK-UHFFFAOYAC
Related Products
- Phosphorous acid
- Phosphorous acid tris(2-fluoroethyl-ester)
- Phosphorous acid,2-ethylhexyl diphenyl ester
- Phosphorous acid,bis(2-ethylhexyl) phenyl ester
- Phosphorous acid,didecyl phenyl ester
- Phosphorous acid,diisodecyl phenyl ester
- Phosphorous acid,diisooctyl 4-octylphenyl ester
- Phosphorous acid,diisooctyl phenyl ester
- Phosphorous acid,dimethyl trimethylsilyl ester
- Phosphorous acid,isooctyl diphenyl ester
- 3620-18-6
- 36204-31-6
- 362045-33-8
- 362045-44-1
- 362049-51-2
- 362049-53-4
- 362049-65-8
- 362051-19-2
- 362052-03-7
- 362-07-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View