-
Name
Prednisone 21-acetate
- EINECS 204-726-0
- CAS No. 125-10-0
- Article Data40
- CAS DataBase
- Density 1.28 g/cm3
- Solubility 23mg/L(25 oC)
- Melting Point 240-242°C (dec.)
- Formula C23H28O6
- Boiling Point 582 °C at 760 mmHg
- Molecular Weight 400.472
- Flash Point 200.2 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R20/21/22; R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Pregna-1,4-diene-3,11,20-trione,17,21-dihydroxy-, 21-acetate (6CI,7CI,8CI);21-Acetoxy-17a-hydroxypregna-1,4-diene-3,11,20-trione;Cortancyl;Delcortin;Delta-Corlin;Deltalone;Ferrosan;NSC 10965;Nisone;Prednisone acetate;D1-Cortisone 21-acetate;D1-Dehydrocortisone acetate;
- PSA 97.74000
- LogP 2.33660
Synthetic route

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In N,N-dimethyl-formamide at 23 - 25℃; for 5h; | 99% |
| With 1-hydroxy-1.oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one pyridinium salt In N,N-dimethyl-formamide at 24 - 28℃; for 4h; | 97% |
| With N-bromoacetamide | |
| With chromium(VI) oxide |
-
-
4224-31-1
21-acetoxy-9-bromo-11β,17-dihydroxy-pregna-1,4-diene-3,20-dione

-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| With tert-Butyl peroxybenzoate Inert atmosphere; Reflux; | 81% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide | |
| With periodic acid | |
| With iodine pentoxide | |
| With Arthrobacter simplex By-2-13 | 17 g |
-
-
52-21-1
prednisolone 21-acetate

-
A
-
898-84-0
(8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-6H-cyclopenta[a]phenanthrene-3,17-dione

-
B
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| Product distribution; Rate constant; Irradiation; radiolytic degradation; |

-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide | |
| With iodine pentoxide; periodic acid |

-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide | |
| With iodine pentoxide; periodic acid | |
| Bromierung und Erhitzen mit 2,4,6-Trimethyl-pyridin; |

| Conditions | Yield |
|---|---|
| In pyridine |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: CrO3 View Scheme |
-
-
28449-43-6
21-acetoxy-11β-hydroxy-pregna-1,4,17(20)c-trien-3-one

-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diacetoxyiodanyl-benzene 2: N-bromo-acetamide View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; manganese(ll) chloride; chromium(VI) oxide / water; chloroform / 30 °C 2: Arthrobacter simplex By-2-13 View Scheme |
-
-
125-10-0
prednisone acetate

-
-
64-19-7
acetic acid

-
-
6677-19-6
17α,21-diacetoxypregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With trifluoroacetic anhydride; toluene-4-sulfonic acid at 0 - 20℃; for 3.5h; | 100% |
| With toluene-4-sulfonic acid; trifluoroacetic anhydride at 0 - 20℃; for 3.5h; Cooling with ice; | 100% |
| With toluene-4-sulfonic acid; trifluoroacetic anhydride at 0 - 20℃; for 3.5h; | 100% |

| Conditions | Yield |
|---|---|
| With di(n-butyl)tin oxide In methanol for 4h; Heating; | 89% |
| Stage #1: prednisone acetate With potassium carbonate In ethanol; water at 15℃; Stage #2: With acetic acid In ethanol; water Solvent; Reagent/catalyst; Temperature; | 68.5% |
| With potassium hydrogencarbonate | |
| With potassium hydroxide | |
| With sodium methylate |
-
-
125-10-0
prednisone acetate

-
-
82423-35-6
21-acetoxy-1,4,16-pregnatriene-3,11,20-trione

| Conditions | Yield |
|---|---|
| Stage #1: prednisone acetate With pyridine; N-chloro-succinimide; sulfur dioxide at 15℃; for 1h; Large scale; Stage #2: With hydrogenchloride In water at 0℃; for 5h; Temperature; Large scale; | 83.5% |
-
-
125-10-0
prednisone acetate

-
-
139016-49-2
21-Acetoxy-1α,2α-epoxy-17α-hydroxy-4-pregnene-1,11,20-trione

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In acetone at 20℃; for 20h; Mechanism; other substrates; methyl(trifluoromethyl)dioxirane as reagent; | 80% |
| With 3,3-dimethyldioxirane In acetone at 20℃; for 20h; | 80% |
-
-
125-10-0
prednisone acetate

-
A
-
139016-49-2
21-Acetoxy-1α,2α-epoxy-17α-hydroxy-4-pregnene-1,11,20-trione

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In dichloromethane; acetone at 20℃; for 20h; | A 80% B 4 % Spectr. C 16 % Spectr. |
| With 3,3-dimethyldioxirane In dichloromethane; acetone at 20℃; for 20h; Title compound not separated from byproducts; | A 80% B 4 % Spectr. C 16 % Spectr. |

-
-
125-10-0
prednisone acetate

-
-
74087-85-7
3,3-dimethyldioxirane

-
-
139016-49-2
21-Acetoxy-1α,2α-epoxy-17α-hydroxy-4-pregnene-1,11,20-trione

| Conditions | Yield |
|---|---|
| In acetone at 23℃; Kinetics; | 73% |
-
-
527-69-5
2-furancarbonyl chloride

-
-
125-10-0
prednisone acetate

-
-
94813-60-2
Furan-2-carboxylic acid (8S,9S,10R,13S,14S,17R)-17-(2-acetoxy-acetyl)-10,13-dimethyl-3,11-dioxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl ester

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane for 72h; | 35% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
125-10-0
prednisone acetate

-
-
111162-85-7
(20Ξ)-20,21-epoxy-17-hydroxy-20-hydroxymethyl-pregna-1,4-diene-3,11-dione

| Conditions | Yield |
|---|---|
| With methanol; diethyl ether |
-
-
507-09-5
thioacetic acid

-
-
125-10-0
prednisone acetate

-
-
124107-03-5
21-acetoxy-1α-acetylsulfanyl-17-hydroxy-pregn-4-ene-3,11,20-trione

| Conditions | Yield |
|---|---|
| Irradiation.mit UV-Licht; |
-
-
125-10-0
prednisone acetate

-
-
102378-22-3
21-acetoxy-17-hydroxy-5,11-dioxo-de-A-pregnane-10aα-carbaldehyde

| Conditions | Yield |
|---|---|
| With ozone | |
| (i) O3, AcOEt, (ii) H2O; Multistep reaction; |
-
-
125-10-0
prednisone acetate

-
-
72011-93-9
21-acetoxy-17-hydroxy-1,5β-cyclo-1,10-seco-pregna-3,9-diene-2,11,20-trione

| Conditions | Yield |
|---|---|
| With 1,4-dioxane Irradiation.UV-Licht; | |
| With ethanol Irradiation.UV-Licht; |
-
-
125-10-0
prednisone acetate

-
-
72779-19-2
21-acetoxy-10,17-dihydroxy-1αH,10αH-1,5-cyclo-5,10-seco-pregn-4-ene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With acetic acid Irradiation.mit UV-Licht; |
-
-
125-10-0
prednisone acetate

-
-
21365-82-2
21-acetoxy-6ξ-bromo-17-hydroxy-pregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide |
-
-
125-10-0
prednisone acetate

-
-
73397-96-3
lumiprednisone 21-acetate

| Conditions | Yield |
|---|---|
| Photoisomerisierung; | |
| With ethanol Irradiation.UV-Licht; |
-
-
125-10-0
prednisone acetate

-
-
114982-44-4
21-acetoxy-1α,5-disulfanediyl-17-hydroxy-5α-pregnane-3,11,20-trione

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen sulfide; sulfur |
-
-
125-10-0
prednisone acetate

-
-
122-52-1
triethyl phosphite

-
-
14413-09-3
O21-Acetyl-1-diaethoxyphosphinyl-cortison

| Conditions | Yield |
|---|---|
| With phenol |
-
-
125-10-0
prednisone acetate

-
-
108-24-7
acetic anhydride

-
-
103004-64-4
3,17,21-Triacetoxy-1-methyl-19-norpregna-1,3.5(10)-trien-11,20-dion

| Conditions | Yield |
|---|---|
| With perchloric acid |
-
-
125-10-0
prednisone acetate

-
-
108-24-7
acetic anhydride

-
-
13396-30-0
1-Methyl-3.11β.17α.21-tetrahydroxy-19-nor-pregna-1.3.5(10).9(11)-tetraen-20-on-tetraacetat

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With perchloric acid |
-
-
125-10-0
prednisone acetate

-
-
14945-34-7
1α,2α,17α-Trihydroxy-21-acetoxy-Δ4-pregnantrion-(3,11,20)

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide | |
| With pyridine; osmium(VIII) oxide |
-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| (i) O3, AcOEt, (ii) H2O, (iii) aq. H3PO3, AcOH; Multistep reaction; |
-
-
125-10-0
prednisone acetate

| Conditions | Yield |
|---|---|
| (i) O3, AcOEt, (ii) H2O; Multistep reaction; |
-
-
125-10-0
prednisone acetate

-
A
-
72011-85-9
21-acetoxy-2,17-dihydroxy-1-methyl-19-nor-pregna-1,3,5(10)-triene-11,20-dione

| Conditions | Yield |
|---|---|
| With 1,4-dioxane Irradiation.UV-Licht; |
Prednisone 21-acetate Specification
The Prednisone 21-acetate, with the CAS registry number 125-10-0, is also known as Prednisone acetate. It belongs to the product categories of Hormone; Biochemical Reagents; Biochemicals. Its EINECS registry number is 204-726-0. This chemical's molecular formula is C23H28O6 and molecular weight is 400.46482. Its IUPAC name is called [2-[(8S,9S,10R,13S,14S,17R)-17-hydroxy-10,13-dimethyl-3,11-dioxo-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl]acetate. This chemical's classification codes are Hormone; Reproductive Effect. It is a kind of adrenal cortical hormone drug and has capabilities of anti-inflammatory, antiallergic. Besides that it is also used for biochemical studies. In addition, it is obtained from acetic acid cortisone via dehydrogenation of selenium dioxide.
Physical properties of Prednisone 21-acetate: (1)ACD/LogP: 2.66; (2)ACD/LogD (pH 5.5): 2.66; (3)ACD/LogD (pH 7.4): 2.66; (4)ACD/BCF (pH 5.5): 61.51; (5)ACD/BCF (pH 7.4): 61.51; (6)ACD/KOC (pH 5.5): 663.95; (7)ACD/KOC (pH 7.4): 663.94; (8)#H bond acceptors: 6; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 5; (11)Index of Refraction: 1.579; (12)Molar Refractivity: 103.66 cm3; (13)Molar Volume: 311.5 cm3; (14)Surface Tension: 54.3 dyne/cm; (15)Density: 1.28 g/cm3; (16)Flash Point: 200.2 °C; (17)Enthalpy of Vaporization: 99.92 kJ/mol; (18)Boiling Point: 582 °C at 760 mmHg; (19)Vapour Pressure: 5.69E-16 mmHg at 25°C.
Uses of Prednisone 21-acetate: it can be used to produce 21-acetoxy-1α,2α-epoxy-17α-hydroxy-4-pregnene-3,11,20-trione at temperature of 20 °C. This reaction will need reagent dimethyldioxirane and solvent acetone with reaction time of 20 hours. The yield is about 80%.
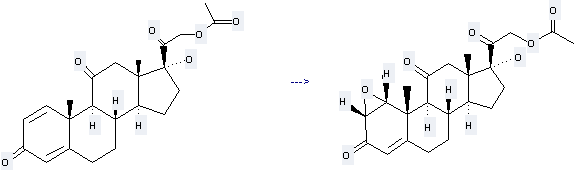
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC(=O)[C@@]3(O)CC[C@H]2[C@@H]4CC\C1=C\C(=O)\C=C/[C@]1(C)[C@H]4C(=O)C[C@@]23C)C
(2)InChI: InChI=1/C23H28O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h6,8,10,16-17,20,28H,4-5,7,9,11-12H2,1-3H3/t16-,17-,20+,21-,22-,23-/m0/s1
(3)InChIKey: MOVRKLZUVNCBIP-RFZYENFJBM
Related Products
- Prednisone
- Prednisone 21-acetate
- 1251002-00-2
- 1251008-65-7
- 1251015-63-0
- 1251018-38-8
- 1251033-27-8
- 125103-95-9
- 125112-68-7
- 12511-31-8
- 125114-77-4
- 125116-23-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View