-
Name
Prednisone
- EINECS 200-160-3
- CAS No. 53-03-2
- Article Data51
- CAS DataBase
- Density 1.3 g/cm3
- Solubility 115mg/L(25 oC)
- Melting Point 236-238 °C(lit.)
- Formula C21H26O5
- Boiling Point 573.7 °C at 760 mmHg
- Molecular Weight 358.434
- Flash Point 314.8 °C
- Transport Information
- Appearance white crystalline powder
- Safety 36/37/39-45-26-16
- Risk Codes 63-34-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, C,
C, F
F
- Synonyms 1,2-Dehydrocortisone;1,4-Pregnadiene-17a,21-diol-3,11,20-trione;1-Dehydrocortisone;17,21-Dihydroxypregn-1,4-diene-3,11,20-trione;17a,21-Dihydroxy-1,4-pregnadiene-3,11,20-trione;Apo-Prednisone;Bicortone;Cartancyl;Colisone;Cortidelt;Dacorten;Decortancyl;Decortin;Dellacort A;Delta-Cortelan;Delta-Dome;Deltacortisone;Deltasone;Deltison;Deltisone;Deltra;Di-Adreson;Pregna-1,4-diene-3,11,20-trione,17,21-dihydroxy-;
- PSA 91.67000
- LogP 1.76580
Synthetic route

| Conditions | Yield |
|---|---|
| With Rhodococcus coprophilus DSM 43347 In dimethyl sulfoxide at 30℃; for 24h; Reagent/catalyst; Green chemistry; regiospecific reaction; | 94% |
| With selenium(IV) oxide | |
| mit Hilfe von Didymella lycopersici; | |
| mit Hilfe von Corynebacterium simplex; | |
| mit Hilfe von Corynebacterium simplex; |

| Conditions | Yield |
|---|---|
| With di(n-butyl)tin oxide In methanol for 4h; Heating; | 89% |
| Stage #1: prednisone acetate With potassium carbonate In ethanol; water at 15℃; Stage #2: With acetic acid In ethanol; water Solvent; Reagent/catalyst; Temperature; | 68.5% |
| With potassium hydrogencarbonate | |
| With potassium hydroxide | |
| With sodium methylate |

| Conditions | Yield |
|---|---|
| With Rhodococcus baikonurensis DSM 44587 In dimethyl sulfoxide at 30℃; for 72h; Reagent/catalyst; Green chemistry; regiospecific reaction; | A 27% B 68% |

| Conditions | Yield |
|---|---|
| With Rhodococcus rhodnii DSM 43960 In dimethyl sulfoxide for 24h; Green chemistry; Enzymatic reaction; | A 30% B 10% |
-
-
50-24-8
prednisolon

-
A
-
898-84-0
(8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-6H-cyclopenta[a]phenanthrene-3,17-dione

-
B
-
53-03-2
Prednison

| Conditions | Yield |
|---|---|
| Product distribution; Rate constant; Irradiation; radiolytic degradation; |
-
-
51192-49-5
Prednisone pivalate

-
-
53-03-2
Prednison

| Conditions | Yield |
|---|---|
| With Curvularia tuberculata In water at 30℃; for 72h; Yield given; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: SeO2 2: methanol.KOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-bromo-acetamide 2: aqueous methanol.KHCO3 View Scheme |
-
-
28449-43-6
21-acetoxy-11β-hydroxy-pregna-1,4,17(20)c-trien-3-one

-
-
53-03-2
Prednison

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: diacetoxyiodanyl-benzene 2: N-bromo-acetamide 3: aqueous methanol.KHCO3 View Scheme |

| Conditions | Yield |
|---|---|
| With oxygen In water; acetonitrile at 24.84℃; Kinetics; Quantum yield; Reagent/catalyst; Photolysis; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetic acid; manganese(ll) chloride; chromium(VI) oxide / water; chloroform / 30 °C 2: Arthrobacter simplex By-2-13 3: potassium carbonate / water; ethanol / 15 °C View Scheme |
-
-
53-03-2
Prednison

-
-
108-24-7
acetic anhydride

-
-
6677-19-6
17α,21-diacetoxypregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With pyridine at 85 - 90℃; for 2.5h; Reagent/catalyst; Temperature; | 98.4% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrazine hydrate In methanol at 62 - 64℃; for 8h; | 98% |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0℃; | 95% |
| Stage #1: Prednison; 4-Nitrophenyl chloroformate In chloroform at 0℃; for 1.25h; Stage #2: With pyridine In chloroform |

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 20℃; Electrochemical reaction; | 89% |
| With manganese(IV) oxide In chloroform for 4h; Heating; | 55.3% |
| Product distribution; Rate constant; Irradiation; radiolytic degradation; | |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 1.17 h / 0 °C / Inert atmosphere 2: sodium periodate / tetrahydrofuran; water; acetone / 3.08 h / 0 - 23 °C / Inert atmosphere View Scheme |
-
-
53-03-2
Prednison

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide for 24h; Ambient temperature; | 86% |
-
-
53-03-2
Prednison

-
-
124-63-0
methanesulfonyl chloride

-
-
76676-23-8
17-hydroxy-21-methanesulfonyloxy-pregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 86% |
-
-
53-03-2
Prednison


| Conditions | Yield |
|---|---|
| With N,N,N′,N′-tetramethyl-N″-tert-butylguanidine In chloroform at 20℃; for 2h; | 86% |
-
-
53-03-2
Prednison

-
-
78261-67-3
17α-hydroxy-11-oxoandrosta-1,4-dien-3-one-17β-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium periodate In tetrahydrofuran; methanol at 20℃; for 2h; | 84% |

| Conditions | Yield |
|---|---|
| With boric acid In ethanol at 20℃; for 72h; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform for 16h; Ambient temperature; | 77% |

| Conditions | Yield |
|---|---|
| With pyridine; iodine; triphenylphosphine In toluene for 6h; Inert atmosphere; Reflux; chemoselective reaction; | 76% |
-
-
50-00-0
formaldehyd

-
-
53-03-2
Prednison

-
-
67254-01-7
(20R)-17,20:20,21-bis[methylenebis(oxy)]pregna-1,4-diene-3,11-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform; water | 73.3% |
-
-
20060-41-7
oleyl hemisuccinate

-
-
53-03-2
Prednison

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 14h; Inert atmosphere; | 71% |
-
-
53-03-2
Prednison

-
-
57099-40-8
17-hydroxy-21-phosphonooxy-pregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With pyridine; 2-cyanoethyl phosphate; dicyclohexyl-carbodiimide | 70% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 0.5h; Molecular sieve; Inert atmosphere; | 60% |
-
-
53-03-2
Prednison

-
-
67067-81-6
21-hydroxy-1,4-pregnadiene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide In acetonitrile for 4h; Ambient temperature; | 54% |
| With trimethylsilyl iodide; sodium sulfite In hexane; chloroform; ethyl acetate; acetonitrile |
-
-
53-03-2
Prednison

-
A
-
118724-36-0
20-hydroxy-3,11-dioxo-1,4,trans-17(20)-pregnatrien-21-al

-
B
-
118724-35-9
20-hydroxy-3,11-dioxo-1,4,cis-17(20)-pregnatrien-21-al

| Conditions | Yield |
|---|---|
| With zinc diacetate; acetic acid Heating; | A 5.1% B 42.2% |

| Conditions | Yield |
|---|---|
| With 2-chloro-1-methyl-pyridinium iodide In acetonitrile at 20℃; for 18h; | 38% |

| Conditions | Yield |
|---|---|
| With 2-chloro-1-methyl-pyridinium iodide In acetonitrile at 20℃; for 18h; | 36% |
-
-
53-03-2
Prednison

-
-
225242-73-9
phenyl(2-trifluoracetamido-2-deoxy-3,4,6-tri-O-acetyl-1-thio-D-glucopyranoside)

-
-
1092578-16-9
17α-hydroxy-3,11,20-trioxo-pregnadien-(1,4)-yl-(21)-2-trifluoracetamido-2-deoxy-3,4,6-tri-O-acetyl-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trimethylsilyl trifluoromethanesulfonate In chloroform at 0℃; Molecular sieve; | 26% |
-
-
1092578-22-7
phenyl-4-O-(2-trifluoracetamido-2-deoxy-3,4,6-tri-O-acetyl-D-glucopyranosyl)-2-trifluoracetamido-2-deoxy-3,6-di-O-acetyl-1-thio-D-glucopyranoside

-
-
53-03-2
Prednison

-
-
1092578-23-8
17α-hydroxy-3,11,20-trioxo-pregnadien-(1,4)-yl-(21)-4-O-(2-trifluoracetamido-2-deoxy-3,4,6-tri-O-acetyl-β-D-glucopyranosyl)-2-trifluoracetamido-2-deoxy-3,6-di-O-acetyl-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trimethylsilyl trifluoromethanesulfonate In chloroform at 0℃; Molecular sieve; | 26% |
-
-
53-03-2
Prednison

-
-
563-41-7
semicarbazide hydrochloride

-
-
113927-06-3
17,21-dihydroxy-pregna-1,4-diene-3,11,20-trione-3,20-disemicarbazone

| Conditions | Yield |
|---|---|
| With Rhodotorula longissima |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate | |
| With calonectria decora | |
| With streptomyces griseus | |
| With streptomyces hydrogenating agents |

| Conditions | Yield |
|---|---|
| With pyridine; chloroform |
-
-
53-03-2
Prednison

-
-
123-62-6
propionic acid anhydride

-
-
115322-27-5
17-hydroxy-21-propionyloxy-pregna-1,4-diene-3,11,20-trione

| Conditions | Yield |
|---|---|
| With pyridine |
Prednisone Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 326.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 26 ,1981,p. 293.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 26 ,1981,p. 293.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Studies (ipr); No Evidence: mouse CANCAR Cancer. 40 (1977),1935. ; (ipr); Equivocal Evidence: rat CANCAR Cancer. 40 (1977),1935. . Reported in EPA TSCA Inventory.
Prednisone Specification
1. Introduction of Prednisone
Prednisone is a white crystalline powder and an organic compound. The systematic name of this chemical is 17,21-dihydroxypregna-1,4-diene-3,11,20-trione. The product's categories are Antitumors for Research and Experimental Use; Biochemistry; Hydroxyketosteroids; Steroids; Intermediates & Fine Chemicals; Pharmaceuticals; Steroid and Hormone. It is also named as 1,4-Pregnadiene-17a,21-diol-3,11,20-trione. In addition, the Classification Code of Prednisone is Adrenal Cortex Hormones; Anti-Inflammatory Agents; Antineoplastic Agents; Antineoplastic agents, hormonal; Drug / Therapeutic Agent; Glucocorticoid; Glucocorticoids; Hormones; Hormones, Hormone Substitutes, and Hormone Antagonists; Human Data; Mutation data; Reproductive Effect; Tumor data. Prednisone is practically insoluble in water.
2. Properties of Prednisone
Physical properties about Prednisone are:
(1)ACD/LogP: 1.57; (2)ACD/LogD (pH 5.5): 1.57; (3)ACD/LogD (pH 7.4): 1.57; (4)ACD/BCF (pH 5.5): 9.16; (5)ACD/BCF (pH 7.4): 9.16; (6)ACD/KOC (pH 5.5): 169.92; (7)ACD/KOC (pH 7.4): 169.92; (8)#H bond acceptors: 5; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 69.67 Å2; (12)Index of Refraction: 1.603; (13)Molar Refractivity: 94.08 cm3; (14)Molar Volume: 273.6 cm3; (15)Polarizability: 37.29×10-24cm3; (16)Surface Tension: 58.5 dyne/cm; (17)Density: 1.3 g/cm3; (18)Flash Point: 314.8 °C; (19)Enthalpy of Vaporization: 98.73 kJ/mol; (20)Boiling Point: 573.7 °C at 760 mmHg; (21)Vapour Pressure: 1.51E-15 mmHg at 25°C.
3. Structure Descriptors of Prednisone
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(CO)[C@@]3(O)CC[C@H]2[C@@H]4CC\C1=C\C(=O)\C=C/[C@]1(C)[C@H]4C(=O)C[C@@]23C
(2)InChI: InChI=1/C21H26O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-15,18,22,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1
(3)InChIKey: XOFYZVNMUHMLCC-ZPOLXVRWBN
(4)Std. InChI: InChI=1S/C21H26O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-15,18,22,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1
(5)Std. InChIKey: XOFYZVNMUHMLCC-ZPOLXVRWSA-N
4. Safety information of Prednisone
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | LDLo | oral | 400ug/kg (0.4mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Irish Journal of Medical Science. Vol. 155, Pg. 234, 1986. |
| man | TDLo | oral | 857ug/kg (0.857mg/kg) | PERIPHERAL NERVE AND SENSATION: SENSORY CHANGE INVOLVING PERIPHERAL NERVE | Neurology. Vol. 36, Pg. 729, 1986. |
| mouse | LD50 | intramuscular | 600mg/kg (600mg/kg) | Cancer Research. Vol. 42, Pg. 122, 1982. | |
| mouse | LD50 | intraperitoneal | 135mg/kg (135mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | subcutaneous | 101mg/kg (101mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| women | TDLo | oral | 2400ug/kg/2D- (2.4mg/kg) | PERIPHERAL NERVE AND SENSATION: SENSORY CHANGE INVOLVING PERIPHERAL NERVE | Neurology. Vol. 36, Pg. 729, 1986. |
| women | TDLo | unreported | 113mg/kg (113mg/kg) | BLOOD: HEMORRHAGE SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | JAMA, Journal of the American Medical Association. Vol. 243, Pg. 1260, 1980. |
5. Safety information of Prednisone
Hazard Codes: Xn,C,F
Risk Statements: 63-34-11
63: Possible risk of harm to the unborn child.
34: Causes burns.
11: Highly Flammable.
Safety Statements: 36/37/39-45-26-16
36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
16: Keep away from sources of ignition - No smoking.
WGK Germany: 3
RTECS: TU4154100
HazardClass: IRRITANT
When you are using this chemical, please be cautious about it as the following:
It is highly flammable and can cause burns. Please keep away from sources of ignition - No smoking. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is possible risk of harm to the unborn child. When you are using it, wear suitable gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
6. Uses of Prednisone
Prednisone can be used to produce androsta-1,4-diene-3,11,17-trione by heating. It will need reagent MnO2 and solvent CHCl3 with reaction time of 4 hours. The yield is about 55.3%.
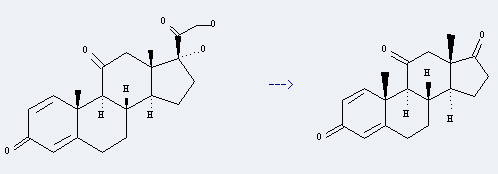
Besides, it is a white crystalline powder, which should be used in biochemical research. Adrenal cortex hormones drugs, it's anti-inflammatory, anti-allergy and commonly used in chemotherapy, you can improve patient general, and it can be used in acute leukemia and other tumors.
7. Production of Prednisone
Prednisone can be got from CORTISONE.
Related Products
- Prednisone
- Prednisone 21-acetate
- 530-35-8
- 5303-65-1
- 530-36-9
- 53038-41-8
- 5304-21-2
- 5304-34-7
- 530-43-8
- 530-44-9
- 530-46-1
- 53046-98-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View