-
Name
Side chain for meropenem
- EINECS 619-190-7
- CAS No. 96034-64-9
- Article Data11
- CAS DataBase
- Density 1.36 g/cm3
- Solubility
- Melting Point 117.0 to 121.0 °C
- Formula C15H19N3O5S
- Boiling Point 555.8 °C at 760 mmHg
- Molecular Weight 353.399
- Flash Point 289.9 °C
- Transport Information
- Appearance White crystalline solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms DMP;(2S,4S) -2-(dimethylaminocarbonyl)-4-mercapto-1- (p-nitrobenzyloxycarbonyl) -1-pyrrolidine;Meropenem Side Chain;(2S,4S)-2-Dimethylaminocarbonyl-4-mercapto-1-(p-nitrobenzyloxycarbonyl)-pyrrolidine;Side chain for meropenem(2S-CIS);2-Dimethylcarbamoyl-4-mercapto-pyrrolidine-1-carboxylic acid 4-nitro-benzyl ester;(2s-cis)-2-[(dimethylamino)carbonyl]-4-mercapto-1-pyrrolidinecarboxylic acid (4-nitrophenyl) methyl ester;(2s,4s)-2-(dimethylaminocarbonyl)-4-mercapto-1-(4'-nitrobenzyloxycarbonyl)-1-pyrrolidine;2s,4s)-2-dimethylaminocarbonyl-4-mercapto-1-p-nitrobenzyloxycabonyl-pyrrolidine;
- PSA 134.47000
- LogP 2.15340
Synthetic route
-
-
127966-47-6
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-mercaptopyrrolidine

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-mercaptopyrrolidine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 5℃; for 0.166667h; Stage #2: N,N-dimethylammonium chloride In N,N-dimethyl-formamide at 0 - 5℃; for 3h; Concentration; | 92.3% |
| Stage #1: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-mercaptopyrrolidine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 5℃; for 0.166667h; Stage #2: N,N-dimethylammonium chloride In N,N-dimethyl-formamide at 0 - 5℃; for 3h; | 92.3% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
151072-00-3
(2S,4S)-5-p-nitrobenzyloxycarbonyl-2-thia-5-azabicyclo<2.2.1>heptan-3-one

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 5h; Ambient temperature; | 92% |
-
-
936226-36-7
bi[(2S,4S)-2-dimethylaminocarbonyl-1-PNZ-pyrrolindin-4-yl]disulfide

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| With tributylphosphine; water In tetrahydrofuran for 0.5h; | 90% |
| With tributylphosphine In tetrahydrofuran; water for 0.5h; | 90% |
-
-
96034-61-6
4-Nitrobenzyl (2S,4S)-4-(acetylthio)-2-[(dimethylamino)carbonyl]pyrrolidine-1-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| With acetyl chloride In methanol at 25 - 30℃; for 6h; | 80% |
| With methanol; acetyl chloride at 25℃; for 2.5 - 5h; Product distribution / selectivity; Heating / reflux; | 70% |
| With Lewatit K 2649 - ion exchange resin In methanol at 25 - 67℃; Product distribution / selectivity; |
-
-
96034-57-0
(2S,4R)-4-Hydroxy-1-(4-nitrobenzyloxycarbonyl)pyrrolidine-2-carboxylic acid

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine; isopropyl chloroformate / dichloromethane / -30 - -20 °C 2.1: triethylamine / dichloromethane / -20 °C 3.1: N,N-dimethyl-formamide / 70 - 80 °C 4.1: sodium hydroxide; water / methanol / 5 - 10 °C 4.2: pH 2 View Scheme |
-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydroxide / water / 0 - 5 °C 1.2: 0 - 5 °C 1.3: 0 - 5 °C 2.1: triethylamine; isopropyl chloroformate / dichloromethane / -30 - -20 °C 3.1: triethylamine / dichloromethane / -20 °C 4.1: N,N-dimethyl-formamide / 70 - 80 °C 5.1: sodium hydroxide; water / methanol / 5 - 10 °C 5.2: pH 2 View Scheme |
-
-
51-35-4
4R-4-hydroxyproline

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydroxide / water / 0 - 5 °C 1.2: 0 - 5 °C 1.3: 0 - 5 °C 2.1: triethylamine; isopropyl chloroformate / dichloromethane / -30 - -20 °C 3.1: triethylamine / dichloromethane / -20 °C 4.1: N,N-dimethyl-formamide / 70 - 80 °C 5.1: sodium hydroxide; water / methanol / 5 - 10 °C 5.2: pH 2 View Scheme | |
| Multi-step reaction with 4 steps 1.1: tributylphosphine / tetrahydrofuran / 25 h / 60 - 65 °C 2.1: diethylamino-sulfur trifluoride / 1,2-dichloro-ethane / 26 h / 0 - 60 °C 3.1: sodium carbonate / acetone; water / 5 h / 0 - 50 °C 4.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 0.17 h / 0 - 5 °C 4.2: 3 h / 0 - 5 °C View Scheme |
-
-
130625-69-3
(2S,4R)-1-p-Nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-hydroxypyrrolidine

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / -20 °C 2.1: N,N-dimethyl-formamide / 70 - 80 °C 3.1: sodium hydroxide; water / methanol / 5 - 10 °C 3.2: pH 2 View Scheme |
-
-
130625-70-6
(2S,4R)-2-dimethylaminocarbonyl-4-mesyloxy-1-p-nitrobenzyloxycarbonylpyrrolidine

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: N,N-dimethyl-formamide / 70 - 80 °C 2.1: sodium hydroxide; water / methanol / 5 - 10 °C 2.2: pH 2 View Scheme |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: diethylamino-sulfur trifluoride / ethylenediamine / 26 h / 0 - 60 °C 2.1: sodium carbonate / acetone; water / 0.25 h / 0 - 25 °C 3.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 0.17 h / 0 - 5 °C 3.2: 3 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: diethylamino-sulfur trifluoride / 1,2-dichloro-ethane / 26 h / 0 - 60 °C 2.1: sodium carbonate / acetone; water / 5 h / 0 - 50 °C 3.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 0.17 h / 0 - 5 °C 3.2: 3 h / 0 - 5 °C View Scheme |
-
-
3398-22-9
trans-4-hydroxyproline

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: tributylphosphine / tetrahydrofuran / 25 h / 60 - 65 °C 2.1: diethylamino-sulfur trifluoride / ethylenediamine / 26 h / 0 - 60 °C 3.1: sodium carbonate / acetone; water / 0.25 h / 0 - 25 °C 4.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 0.17 h / 0 - 5 °C 4.2: 3 h / 0 - 5 °C View Scheme |
-
-
93711-81-0, 90776-59-3
(4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-02-1
p-nitrobenzyl (4R,5S,6S)-3-<(3S,5S)-5-dimethylaminocarbonyl-1-(p-nitrobenzyloxycarbonyl)pyrrolidin-3-ylthio>-6-<(1R)-1-hydroxyethyl>-4-methyl-7-oxo-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at -15 - -10℃; for 5 - 8h; | 91.3% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at -10℃; for 3.16667h; Product distribution / selectivity; | 18% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at -10℃; for 3.16667h; Product distribution / selectivity; | 17% |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
936226-36-7
bi[(2S,4S)-2-dimethylaminocarbonyl-1-PNZ-pyrrolindin-4-yl]disulfide

| Conditions | Yield |
|---|---|
| With iron(III) chloride; oxygen In methanol at 25℃; under 750.075 Torr; for 4h; Pressure; Temperature; Reagent/catalyst; | 90% |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (p-nitrophenyl)methyl 6-(1-hydroxyethyl)-5-methyl-3,7-dioxoazabicyclo[3.2.0]heptane-2-carboxylate With N-ethyl-N,N-diisopropylamine; Diphenylphosphinic chloride In acetonitrile at -35℃; for 0.5h; Stage #2: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetonitrile | 85% |
-
-
100207-82-7
bromomagnesium N-isopropyl-N-cyclohexylamide

-
-
158168-61-7
4-nitrobenzyl (1 R , 5 S , 6 S)-2-(2-diethylcarbamoylphenylsulphinyl)-1-methyl-6-[1(R)-trimethylsilyloxyethyl]-1-carbapen-2-em-3-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
105344-45-4
p-nitrobenzyl (4R,5S,6S)-3-<(3S,5S)-5-dimethylaminocarbonyl-1-(p-nitrobenzyloxycarbonyl)pyrrolidin-3-ylthio>-4-methyl-7-oxo-6-<(1R)-1-trimethylsilyloxoethyl>-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| With sodium chloride; ammonium chloride In tetrahydrofuran; (2S)-N-methyl-1-phenylpropan-2-amine hydrate | 78% |


-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (p-nitrophenyl)methyl 5-ethyl-6-(1-hydroxyethyl)-3,7-dioxoazabicyclo[3.2.0]heptane-2-carboxylate With N-ethyl-N,N-diisopropylamine; Diphenylphosphinic chloride In acetonitrile at -35℃; Stage #2: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetonitrile | 77% |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
158168-65-1
(4R,5S,6S)-6-[(R)-1-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-3-(2-diethylcarbamoyl-benzenesulfonyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid 4-nitro-benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; magnesium bromide In tetrahydrofuran for 19h; Ambient temperature; | 73% |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-nitrobenzyl 2-diazo-4-((2R,3S)-3-((R)-1-(methylsulfonamido)ethyl)-4-oxoazetidin-2-yl)-3-oxopentanoate With rhodium (II) octanoate dimer In acetone for 1h; Reflux; Stage #2: With dmap; N-ethyl-N,N-diisopropylamine; chlorophosphoric acid diphenyl ester In acetone at -50 - 20℃; for 0.5h; Stage #3: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetone at -20 - 0℃; for 4h; | 48% |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (2R,5R,6S)-5-methyl-4-nitrobenzyl ester-6-((R)-1-hydroxyethyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylic acid With N-ethyl-N,N-diisopropylamine; Diphenylphosphinic chloride In acetonitrile at -40℃; for 0.333333h; Stage #2: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetonitrile at -40 - -10℃; for 0.5h; | 35% |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine


| Conditions | Yield |
|---|---|
| Stage #1: {(S)-2-[(R)-1-(4-Chloro-phenylsulfanylcarbonyl)-ethyl]-4-oxo-azetidin-1-yl}-acetic acid 4-nitro-benzyl ester With sodium hydride; allyl bromide In tetrahydrofuran at -40℃; Cyclization; Stage #2: With chlorophosphoric acid diphenyl ester at -20℃; Substitution; Stage #3: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N,N-diethyl-N-isopropylamine; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 0℃; Substitution; | 21% |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: C15H14N2O7 With N-ethyl-N,N-diisopropylamine; Diphenylphosphinic chloride In acetonitrile at -40℃; for 0.75h; Stage #2: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetonitrile for 1.5h; | 15% |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
161692-28-0
(4R,5R,6S,8R)-p-nitrobenzyl-3-(diphenylphosphoryloxy)-4-methyl-6-(1-(trimethylsilyloxy)ethyl)-7-oxo-1-azabicyclo<3.2.0>hept-2-ene-2-carboxylate

-
-
105344-45-4
p-nitrobenzyl (4R,5S,6S)-3-<(3S,5S)-5-dimethylaminocarbonyl-1-(p-nitrobenzyloxycarbonyl)pyrrolidin-3-ylthio>-4-methyl-7-oxo-6-<(1R)-1-trimethylsilyloxoethyl>-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran; toluene for 2h; Yield given; |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
105318-43-2
p-nitrobenzyl (4R,5R,6S)-6-<(1R)-1-tert-butyldimethylsilyloxyethyl>-3-diphenylphosphoryloxy-4-methyl-7-oxo-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran; toluene for 2h; Yield given; |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
158168-62-8
(4R,5S,6S)-6-[(R)-1-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-3-(2-diethylcarbamoyl-benzenesulfinyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid 4-nitro-benzyl ester

| Conditions | Yield |
|---|---|
| With bromomagnesium N-isopropyl-N-cyclohexylamide 1.) THF, 0 deg C, 5 min, 2.) THF, 0 deg C, 30 min; Yield given. Multistep reaction; |
-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: NaH; allylbromide / tetrahydrofuran / -40 °C 1.2: diphenyl chlorophosphate / -20 °C 1.3: 21 percent / i-PrEt2N; DBU / acetonitrile / 0 °C 2.1: 24 percent / H2 / Pd-C / tetrahydrofuran; aq. phosphate buffer View Scheme |
-
-
93711-81-0, 90776-59-3
(4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: (4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine In N,N-dimethyl-formamide at -50 - 20℃; for 1h; Stage #2: With 4-methyl-morpholine; hydrogen; acetic acid; 5%-palladium/activated carbon In ethyl acetate at 20 - 25℃; for 3h; pH=~ 7; Product distribution / selectivity; Aqueous buffer; |
-
-
93711-81-0, 90776-59-3
(4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
141818-52-2
4-nitrobenzyl (4R,5S,6S)-3-[[(3S,5S)-5-(dimethylcarbamoyl)-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxy-ethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate

| Conditions | Yield |
|---|---|
| With diisopropylamine In N,N-dimethyl-formamide at -50 - 20℃; for 1h; |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-03-2
meropenem

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitrobenzyl (4R,5R,6S)-3-[(diphenoxyphosphoryl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at -50 - 25℃; for 1h; Stage #2: With 4-methyl-morpholine; hydrogen; acetic acid; 5%-palladium/activated carbon In water; ethyl acetate; N,N-dimethyl-formamide at 20 - 25℃; for 3h; pH=~ 7; |
-
-
93711-81-0, 90776-59-3
(4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-03-2
meropenem

| Conditions | Yield |
|---|---|
| Stage #1: (4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one; water at -20 - -5℃; Stage #2: With 4-methyl-morpholine; hydrogenchloride; 5% Pd(II)/C(eggshell); hydrogen In 1-methyl-pyrrolidin-2-one; water at 25℃; pH=6.5 - 7; | |
| Stage #1: (4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one at -20 - -5℃; Stage #2: With hydrogen; 5%-palladium/activated carbon In 1-methyl-pyrrolidin-2-one at 25℃; pH=6.5 - 7; N-methylmorpholine - hydrochloric acid - buffer; | |
| Stage #1: (4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 0 - 5℃; for 5h; Stage #2: With potassium dihydrogenphosphate; zinc In water; ethyl acetate at 25 - 35℃; for 1h; | |
| Stage #1: (4-nitrophenyl)methyl (4R,5R,6S)-3-[(diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate; (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In ISOPROPYLAMIDE at 0 - 5℃; for 5h; Stage #2: With hydrogenchloride In water; ethyl acetate Stage #3: With potassium dihydrogenphosphate In water; ethyl acetate at 25 - 35℃; for 1h; |
-
-
104873-15-6
4-nitrobenzyl (4R,5R,6S)-6-[(1R)-1-hydroxyethyl]-4-methyl-3,7-dioxo-1-azabicyclo[3.2.0]-heptane-2-carboxylate

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-02-1
p-nitrobenzyl (4R,5S,6S)-3-<(3S,5S)-5-dimethylaminocarbonyl-1-(p-nitrobenzyloxycarbonyl)pyrrolidin-3-ylthio>-6-<(1R)-1-hydroxyethyl>-4-methyl-7-oxo-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitrobenzyl (4R,5R,6S)-6-[(1R)-1-hydroxyethyl]-4-methyl-3,7-dioxo-1-azabicyclo[3.2.0]-heptane-2-carboxylate With N-ethyl-N,N-diisopropylamine; chlorophosphoric acid diphenyl ester In acetonitrile at 10 - 15℃; for 5h; Stage #2: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine With N-ethyl-N,N-diisopropylamine In acetonitrile at 10 - 15℃; for 5 - 8h; |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-03-2
meropenem

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / -35 - -30 °C 2: hydrogen; 2,6-dimethylpyridine / palladium 10% on activated carbon / water; tetrahydrofuran / 20 - 25 °C / 13501.4 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / -35 - -30 °C 2: hydrogen; 2,6-dimethylpyridine / palladium 10% on activated carbon / dichloromethane; water; tetrahydrofuran / 20 - 25 °C / 13501.4 Torr View Scheme |

-
-
96034-64-9
(2S,4S)-1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl-4-thiolpyrrolidine

-
-
96036-02-1
p-nitrobenzyl (4R,5S,6S)-3-<(3S,5S)-5-dimethylaminocarbonyl-1-(p-nitrobenzyloxycarbonyl)pyrrolidin-3-ylthio>-6-<(1R)-1-hydroxyethyl>-4-methyl-7-oxo-1-azabicyclo<3.2.0>hept-2-en-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -35 - -30℃; | |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -35 - -30℃; |
Side chain for Meropenem Chemical Properties
Molecular Structure of Side chain for Meropenem (CAS NO.96034-64-9):
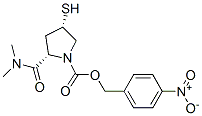
IUPAC Name: 3-chloro-10-methylacridin-9-one
Empirical Formula: C15H19N3O5S
Molecular Weight: 353.3935
H bond acceptors: 8
H bond donors: 0
Freely Rotating Bonds: 6
Molar Refractivity: 89.96 cm3
Molar Volume: 258.9 cm3
Surface Tension: 61.1 dyne/cm
Index of Refraction: 1.611
Surface Tension: 61.1 dyne/cm
Density: 1.36 g/cm3
Flash Point: 289.9 °C
Enthalpy of Vaporization: 83.72 kJ/mol
Boiling Point: 555.8 °C at 760 mmHg
Vapour Pressure: 2.17E-12 mmHg at 25°C
Appearance:White Crystalline Solid
Product Categories: Chemical intermediate for Meropenem; intermediatesofmeropenam; Organic acids; API intermediates;(intermediate of meropenem); (the sidechain of meropenem); Intermediates & Fine Chemicals; Pharmaceuticals; Pharmaceutical; pharmaceutical intermediates
Synonyms of Side chain for Meropenem (CAS NO.96034-64-9): 2-Dimethylcarbamoyl-4-mercapto-pyrrolidine-1-carboxylic acid 4-nitro-benzyl ester ; (2s-cis)-2-[(dimethylamino)carbonyl]-4-mercapto-1-pyrrolidinecarboxylic acid (4-nitrophenyl) methyl ester ; (2s,4s)-2-(dimethylaminocarbonyl)-4-mercapto-1-(4'-nitrobenzyloxycarbonyl)-1-pyrrolidine ; (2s,4s)-2-dimethylaminocarbonyl-4-mercapto-1-p-nitrobenzyloxycabonyl-pyrrolidine ; (2s,4s)-2-(dimethylaminocarbonyl)-4-mercapto-1-(p-nitrobenzyloxycarbonyl)-1-pyrrolidine ; side chain for meropenem ; (2s,4s)-2-(dimethylaminocarbonyl)-4-mercapto-1- (p-nitrobenzyloxycarbonyl)-1- py ; (2s,4s)-2-dimethylcarbamoyl-4-mercapto-pyrrolidine-1-carboxylic acid 4-nitro-benzyl ester
Side chain for Meropenem Specification
Side chain for Meropenem (CAS NO.96034-64-9) is a white Crystalline Solid, Side chain for Meropenem (96034-64-9) is an intermediate used for the synthesis of Meropenem.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View