-
Name
SPINOSAD
- EINECS 620-162-1
- CAS No. 131929-60-7
- Article Data2
- CAS DataBase
- Density 1.167 g/cm3
- Solubility Spinosyn A: 290 mg l-1 (pH 5); Spinosyn D: 29 mg l-1 (pH 5)
- Melting Point 84oC to 99.5oC
- Formula C41H65NO10
- Boiling Point 801.515 °C at 760 mmHg
- Molecular Weight 731.968
- Flash Point 438.541 °C
- Transport Information UN 3077
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (-)-SpinosynA;A 83543A;Lepicidin A;Spinosyn A;
- PSA 111.22000
- LogP 5.48740
Synthetic route

-
-
131929-68-5
(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-9-ethyl-13-hydroxy-14-methyl-2-(((2R,3R,5S,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-3,3a,5b,6,9,10,11,12,13,14,16a,16b-dodecahydro-1H-as-indaceno[3,2-d][1]oxacyclododecine-7,15(2H,5aH)-dione

-
A
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| With silver trifluoromethanesulfonate In dichloromethane for 3h; Yield given. Yields of byproduct given; |

-
-
6026-86-4
methyl 4-formylbutanoate

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: (-)-(1S,2R)-N,N-dibutylnorephedrine / hexane; toluene / 72 h / 0 °C 2: 84 percent / 2,6-lutidine / CH2Cl2 / 2 h / Ambient temperature 3: 94 percent / DIBAL-H / CH2Cl2; toluene / 0.5 h / -78 °C 4: 1.) Bu2BOTf, Et3N / 1.) CH2Cl2, from 0 to 5 deg C, 20 min, 2.) CH2Cl2, from -78 to 0 deg C, 3 h 5: 1.41 g / DMAP / CH2Cl2 / 144 h / Ambient temperature 6: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 7: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 8: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 9: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 10: Jones reagent / acetone / 1.5 h / 0 °C 11: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 12: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 13: 63 percent / methanolic K2CO3 / 48 h / Heating 14: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 15: 91 percent / 49percent aq. HF / acetonitrile / 24 h 16: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
56587-30-5
D-forosamine

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) diethyl azodicarboxylate, PPh3 / 1.) THF, -50 deg C, 10 min, 2.) THF, from -50 to 20 deg C, 3 h 2: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
118545-01-0
(S)-5-Hydroxy-heptanoic acid methyl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 84 percent / 2,6-lutidine / CH2Cl2 / 2 h / Ambient temperature 2: 94 percent / DIBAL-H / CH2Cl2; toluene / 0.5 h / -78 °C 3: 1.) Bu2BOTf, Et3N / 1.) CH2Cl2, from 0 to 5 deg C, 20 min, 2.) CH2Cl2, from -78 to 0 deg C, 3 h 4: 1.41 g / DMAP / CH2Cl2 / 144 h / Ambient temperature 5: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 6: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 7: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 8: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 9: Jones reagent / acetone / 1.5 h / 0 °C 10: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 11: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 12: 63 percent / methanolic K2CO3 / 48 h / Heating 13: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 14: 91 percent / 49percent aq. HF / acetonitrile / 24 h 15: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
155189-82-5
13,14β-Dihydro A83543A aglycon 9α,17α-bis-O-(TBDMS) ether

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 19 steps 1: t-BuOOH, Triton B / methanol; H2O; tetrahydrofuran / 120 h / Ambient temperature 2: diethyl ether / 0 °C 3: 87 percent / LiAlH4 / diethyl ether / 1 h / Ambient temperature 4: 85 percent / NaIO4 / tetrahydrofuran; H2O / Ambient temperature 5: 1.) KHMDS / 1.) THF, toluene, a) -78 deg C, 15 min, b) RT, 1.5 h, 2.) THF, toluene, from 0 to 20 deg C, 2 h 6: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 7: 90 percent / pyridine, DMAP 8: MeOH / 168 h / Heating 9: camphorsulfonic acid / CH2Cl2 / 24 h 10: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 11: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 12: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 13: Jones reagent / acetone / 1.5 h / 0 °C 14: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 15: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 16: 63 percent / methanolic K2CO3 / 48 h / Heating 17: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 18: 91 percent / 49percent aq. HF / acetonitrile / 24 h 19: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-35-1
(S)-5-(tert-butyldimethylsiloxy)heptanal

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 1.) Bu2BOTf, Et3N / 1.) CH2Cl2, from 0 to 5 deg C, 20 min, 2.) CH2Cl2, from -78 to 0 deg C, 3 h 2: 1.41 g / DMAP / CH2Cl2 / 144 h / Ambient temperature 3: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 4: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 5: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 6: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 7: Jones reagent / acetone / 1.5 h / 0 °C 8: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 9: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 10: 63 percent / methanolic K2CO3 / 48 h / Heating 11: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 12: 91 percent / 49percent aq. HF / acetonitrile / 24 h 13: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-34-0
(S)-5-(tert-Butyl-dimethyl-silanyloxy)-heptanoic acid methyl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 94 percent / DIBAL-H / CH2Cl2; toluene / 0.5 h / -78 °C 2: 1.) Bu2BOTf, Et3N / 1.) CH2Cl2, from 0 to 5 deg C, 20 min, 2.) CH2Cl2, from -78 to 0 deg C, 3 h 3: 1.41 g / DMAP / CH2Cl2 / 144 h / Ambient temperature 4: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 5: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 6: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 7: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 8: Jones reagent / acetone / 1.5 h / 0 °C 9: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 10: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 11: 63 percent / methanolic K2CO3 / 48 h / Heating 12: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 13: 91 percent / 49percent aq. HF / acetonitrile / 24 h 14: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-32-8
Formic acid 2-((3S,3aR,5aS,7R,8aR,8bS)-7-hydroxy-2-oxo-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-as-indacen-3-yl)-ethyl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 90 percent / pyridine, DMAP 2: MeOH / 168 h / Heating 3: camphorsulfonic acid / CH2Cl2 / 24 h 4: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 5: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 6: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 7: Jones reagent / acetone / 1.5 h / 0 °C 8: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 9: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 10: 63 percent / methanolic K2CO3 / 48 h / Heating 11: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 12: 91 percent / 49percent aq. HF / acetonitrile / 24 h 13: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-30-6
(2R,3aS,5aR,5bS,10aS,10bR)-2-(tert-Butyl-dimethyl-silanyloxy)-2,3,3a,5a,5b,6,7,9,9a,10,10a,10b-dodecahydro-1H-8-oxa-cyclopenta[a]fluoren-9-ol

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 1.) KHMDS / 1.) THF, toluene, a) -78 deg C, 15 min, b) RT, 1.5 h, 2.) THF, toluene, from 0 to 20 deg C, 2 h 2: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 3: 90 percent / pyridine, DMAP 4: MeOH / 168 h / Heating 5: camphorsulfonic acid / CH2Cl2 / 24 h 6: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 7: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 8: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 9: Jones reagent / acetone / 1.5 h / 0 °C 10: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 11: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 12: 63 percent / methanolic K2CO3 / 48 h / Heating 13: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 14: 91 percent / 49percent aq. HF / acetonitrile / 24 h 15: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: camphorsulfonic acid / CH2Cl2 / 24 h 2: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 3: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 4: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 5: Jones reagent / acetone / 1.5 h / 0 °C 6: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 7: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 8: 63 percent / methanolic K2CO3 / 48 h / Heating 9: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 10: 91 percent / 49percent aq. HF / acetonitrile / 24 h 11: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-33-9
2,2-Dimethyl-propionic acid (2R,3aS,5aR,6S,8aS,8bR)-6-(2-formyloxy-ethyl)-7-oxo-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-as-indacen-2-yl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: MeOH / 168 h / Heating 2: camphorsulfonic acid / CH2Cl2 / 24 h 3: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 4: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 5: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 6: Jones reagent / acetone / 1.5 h / 0 °C 7: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 8: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 9: 63 percent / methanolic K2CO3 / 48 h / Heating 10: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 11: 91 percent / 49percent aq. HF / acetonitrile / 24 h 12: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-36-2
(R)-3-[(2R,3S,7S)-7-(tert-Butyl-dimethyl-silanyloxy)-3-hydroxy-2-methyl-nonanoyl]-4-isopropyl-oxazolidin-2-one

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 1.41 g / DMAP / CH2Cl2 / 144 h / Ambient temperature 2: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 3: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 4: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 5: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 6: Jones reagent / acetone / 1.5 h / 0 °C 7: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 8: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 9: 63 percent / methanolic K2CO3 / 48 h / Heating 10: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 11: 91 percent / 49percent aq. HF / acetonitrile / 24 h 12: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204519-32-4
(S)-1-[(3S,3aR,5aS,7R,8aR,8bS)-7-(tert-Butyl-dimethyl-silanyloxy)-3-(2-hydroxy-ethyl)-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-as-indacen-2-yl]-2-methyl-nonane-1,2,7-triol

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: 85 percent / NaIO4 / tetrahydrofuran; H2O / Ambient temperature 2: 1.) KHMDS / 1.) THF, toluene, a) -78 deg C, 15 min, b) RT, 1.5 h, 2.) THF, toluene, from 0 to 20 deg C, 2 h 3: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 4: 90 percent / pyridine, DMAP 5: MeOH / 168 h / Heating 6: camphorsulfonic acid / CH2Cl2 / 24 h 7: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 8: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 9: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 10: Jones reagent / acetone / 1.5 h / 0 °C 11: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 12: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 13: 63 percent / methanolic K2CO3 / 48 h / Heating 14: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 15: 91 percent / 49percent aq. HF / acetonitrile / 24 h 16: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-37-3
(2R,3S,7S)-7-(tert-Butyl-dimethyl-silanyloxy)-3-(tert-butyl-diphenyl-silanyloxy)-2-methyl-nonanoic acid

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 2: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 3: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 4: Jones reagent / acetone / 1.5 h / 0 °C 5: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 6: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 7: 63 percent / methanolic K2CO3 / 48 h / Heating 8: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 9: 91 percent / 49percent aq. HF / acetonitrile / 24 h 10: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-40-8
(2R,3S,7S)-7-(tert-Butyl-dimethyl-silanyloxy)-3-(tert-butyl-diphenyl-silanyloxy)-2-methyl-nonanoyl chloride

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 2: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 3: Jones reagent / acetone / 1.5 h / 0 °C 4: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 5: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 6: 63 percent / methanolic K2CO3 / 48 h / Heating 7: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 8: 91 percent / 49percent aq. HF / acetonitrile / 24 h 9: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-31-7
(3S,3aR,5aS,7R,8aR,8bS)-7-(tert-Butyl-dimethyl-silanyloxy)-3-[2-(tert-butyl-dimethyl-silanyloxy)-ethyl]-2-[1-(tert-butyl-dimethyl-silanyloxy)-meth-(E)-ylidene]-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-as-indacene

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 2: 90 percent / pyridine, DMAP 3: MeOH / 168 h / Heating 4: camphorsulfonic acid / CH2Cl2 / 24 h 5: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 6: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 7: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 8: Jones reagent / acetone / 1.5 h / 0 °C 9: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 10: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 11: 63 percent / methanolic K2CO3 / 48 h / Heating 12: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 13: 91 percent / 49percent aq. HF / acetonitrile / 24 h 14: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1: diethyl ether / 0 °C 2: 87 percent / LiAlH4 / diethyl ether / 1 h / Ambient temperature 3: 85 percent / NaIO4 / tetrahydrofuran; H2O / Ambient temperature 4: 1.) KHMDS / 1.) THF, toluene, a) -78 deg C, 15 min, b) RT, 1.5 h, 2.) THF, toluene, from 0 to 20 deg C, 2 h 5: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 6: 90 percent / pyridine, DMAP 7: MeOH / 168 h / Heating 8: camphorsulfonic acid / CH2Cl2 / 24 h 9: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 10: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 11: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 12: Jones reagent / acetone / 1.5 h / 0 °C 13: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 14: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 15: 63 percent / methanolic K2CO3 / 48 h / Heating 16: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 17: 91 percent / 49percent aq. HF / acetonitrile / 24 h 18: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 87 percent / LiAlH4 / diethyl ether / 1 h / Ambient temperature 2: 85 percent / NaIO4 / tetrahydrofuran; H2O / Ambient temperature 3: 1.) KHMDS / 1.) THF, toluene, a) -78 deg C, 15 min, b) RT, 1.5 h, 2.) THF, toluene, from 0 to 20 deg C, 2 h 4: 1.) MCPBA, NaHCO3, 2.) TBAF, 3.) NaIO4 5: 90 percent / pyridine, DMAP 6: MeOH / 168 h / Heating 7: camphorsulfonic acid / CH2Cl2 / 24 h 8: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 9: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 10: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 11: Jones reagent / acetone / 1.5 h / 0 °C 12: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 13: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 14: 63 percent / methanolic K2CO3 / 48 h / Heating 15: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 16: 91 percent / 49percent aq. HF / acetonitrile / 24 h 17: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 30percent aq. H2O2, LiOH / H2O; tetrahydrofuran; dimethylformamide / 0 °C 2: oxalyl chloride / dimethylformamide; hexane / 0.75 h / Ambient temperature 3: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 4: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 5: Jones reagent / acetone / 1.5 h / 0 °C 6: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 7: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 8: 63 percent / methanolic K2CO3 / 48 h / Heating 9: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 10: 91 percent / 49percent aq. HF / acetonitrile / 24 h 11: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-38-4
2,2-Dimethyl-propionic acid (2R,3aS,5aR,6S,8aS,8bR)-6-[2-(4-methoxy-benzyloxy)-ethyl]-7-trifluoromethanesulfonyloxy-1,2,3,3a,5a,6,8a,8b-octahydro-as-indacen-2-yl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 2: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 3: Jones reagent / acetone / 1.5 h / 0 °C 4: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 5: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 6: 63 percent / methanolic K2CO3 / 48 h / Heating 7: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 8: 91 percent / 49percent aq. HF / acetonitrile / 24 h 9: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-44-2
(2R,3S,3aR,5aS,7R,8aR,8bS)-2-[(2R,3S,7S)-3-(tert-butyldiphenylsiloxy)-7-hydroxy-2-methylnonanoyl]-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-7-hydroxy-as-indacene-3-acetic acid κ-lactone

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 2: 91 percent / 49percent aq. HF / acetonitrile / 24 h 3: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-43-1
(3S,3aR,5aS,7R,8aR,8bS)-2-[(2R,3S,7S)-3-(tert-butyldiphenylsiloxy)-7-hydroxy-2-methylnonanoyl]-3,3a,5a,6,7,8,8a,8b-octahydro-7-hydroxy-as-indacene-3-acetic acid κ-lactone pivalate

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 63 percent / methanolic K2CO3 / 48 h / Heating 2: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 3: 91 percent / 49percent aq. HF / acetonitrile / 24 h 4: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 2: 63 percent / methanolic K2CO3 / 48 h / Heating 3: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 4: 91 percent / 49percent aq. HF / acetonitrile / 24 h 5: AgOTf3 / CH2Cl2 / 3 h View Scheme |

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Jones reagent / acetone / 1.5 h / 0 °C 2: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 3: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 4: 63 percent / methanolic K2CO3 / 48 h / Heating 5: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 6: 91 percent / 49percent aq. HF / acetonitrile / 24 h 7: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-42-0
2,2-Dimethyl-propionic acid (2R,3aS,5aR,6S,8aS,8bR)-7-[(2R,3S,7S)-7-(tert-butyl-dimethyl-silanyloxy)-3-(tert-butyl-diphenyl-silanyloxy)-2-methyl-nonanoyl]-6-carboxymethyl-1,2,3,3a,5a,6,8a,8b-octahydro-as-indacen-2-yl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 2: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 3: 63 percent / methanolic K2CO3 / 48 h / Heating 4: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 5: 91 percent / 49percent aq. HF / acetonitrile / 24 h 6: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-45-3
(3S,3aR,5aS,7S,8aR,8bS)-2-[(2R,3S,7S)-3-(tert-butyldiphenylsiloxy)-7-hydroxy-2-methylnonanoyl]-7-[(6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranosyl)oxy]-3,3a,5a,6,7,8,8a,8b-octahydro-as-indacene-3-acetic acid κ-lactone

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / 49percent aq. HF / acetonitrile / 24 h 2: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-41-9
2,2-Dimethyl-propionic acid (2R,3aS,5aR,6S,8aS,8bR)-7-[(2R,3S,7S)-7-(tert-butyl-dimethyl-silanyloxy)-3-(tert-butyl-diphenyl-silanyloxy)-2-methyl-nonanoyl]-6-[2-(4-methoxy-benzyloxy)-ethyl]-1,2,3,3a,5a,6,8a,8b-octahydro-as-indacen-2-yl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 2: Jones reagent / acetone / 1.5 h / 0 °C 3: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 4: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 5: 63 percent / methanolic K2CO3 / 48 h / Heating 6: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 7: 91 percent / 49percent aq. HF / acetonitrile / 24 h 8: AgOTf3 / CH2Cl2 / 3 h View Scheme |
-
-
204381-17-9
2,2-Dimethyl-propionic acid (2R,3aS,5aR,6S,8aS,8bR)-6-[2-(4-methoxy-benzyloxy)-ethyl]-7-oxo-1,2,3,3a,5a,6,7,8,8a,8b-decahydro-as-indacen-2-yl ester

-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 1.) KHMDS / 1.) toluene, THF, -78 deg C, 30 min, 2.) toluene, THF, from -78 deg C to RT, 4 h 2: 1.) Me3SnSnMe3, (Ph3P)4Pd, LiCl, 2.) Pd2(dba)3*CHCl3, (i-Pr)2NEt / 1.) THF, 60 deg C, 2 d, 2.) benzene, RT, 6 h 3: DDQ, phosphate buffer / CH2Cl2 / 2 h / 0 °C 4: Jones reagent / acetone / 1.5 h / 0 °C 5: aq. AcOH / tetrahydrofuran / 24 h / 0 - 20 °C 6: 2,4,6-trichlorobenzoyl chlorie, DMAP, Et3N / benzene 7: 63 percent / methanolic K2CO3 / 48 h / Heating 8: 85 percent / trityl perchlorate / toluene / 3.17 h / 0 - 20 °C 9: 91 percent / 49percent aq. HF / acetonitrile / 24 h 10: AgOTf3 / CH2Cl2 / 3 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: Tetrabutylammoniumsalz der Diphenylphosphinsaeure; copper(I) thiophene-2-carboxylate; tris-(dibenzylideneacetone)dipalladium(0) / N,N-dimethyl-formamide / 2 h / 20 °C / Inert atmosphere; Schlenk technique 2.1: tributylphosphine / tetrahydrofuran / 6 h / 20 °C / Inert atmosphere 3.1: diisobutylaluminium hydride / dichloromethane / 2 h / -78 °C 4.1: manganese(IV) oxide / dichloromethane / 5 h / 20 °C 5.1: (2R,5R)-(+)-2-tert-butyl-3-methyl-5-benzyl-4-imidazolidinone; trifluoroacetic acid / water; acetonitrile / 72 h / -20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.67 h / -78 °C 6.2: 2 h / -78 °C 6.3: 20 h / -78 - 20 °C 7.1: silver(I) triflimide; [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I); N-iodo-succinimide / acetone; water / 5 h / -15 - 20 °C 8.1: palladium diacetate; tetraethylammonium chloride; trifuran-2-yl-phosphane / toluene / 6 h / 90 °C / 2280.15 Torr / Molecular sieve 9.1: pyridine; hydrogen fluoride / tetrahydrofuran / 0 - 20 °C 10.1: dichloromethane / 1 h / 20 °C / Molecular sieve 10.2: 24 h / -30 °C 11.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 5 h / 0 °C / pH 7 12.1: [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I); silver trifluoromethanesulfonate / chlorobenzene / 25 h / 20 - 50 °C / Molecular sieve View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: tributylphosphine / tetrahydrofuran / 6 h / 20 °C / Inert atmosphere 2.1: diisobutylaluminium hydride / dichloromethane / 2 h / -78 °C 3.1: manganese(IV) oxide / dichloromethane / 5 h / 20 °C 4.1: (2R,5R)-(+)-2-tert-butyl-3-methyl-5-benzyl-4-imidazolidinone; trifluoroacetic acid / water; acetonitrile / 72 h / -20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.67 h / -78 °C 5.2: 2 h / -78 °C 5.3: 20 h / -78 - 20 °C 6.1: silver(I) triflimide; [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I); N-iodo-succinimide / acetone; water / 5 h / -15 - 20 °C 7.1: palladium diacetate; tetraethylammonium chloride; trifuran-2-yl-phosphane / toluene / 6 h / 90 °C / 2280.15 Torr / Molecular sieve 8.1: pyridine; hydrogen fluoride / tetrahydrofuran / 0 - 20 °C 9.1: dichloromethane / 1 h / 20 °C / Molecular sieve 9.2: 24 h / -30 °C 10.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 5 h / 0 °C / pH 7 11.1: [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I); silver trifluoromethanesulfonate / chlorobenzene / 25 h / 20 - 50 °C / Molecular sieve View Scheme |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; sulfuric acid; water In dimethyl sulfoxide at 0℃; for 0.5h; | 97.1% |
-
-
131929-60-7
spinosyn A

-
-
187170-37-2
5,6-dihydrospinosyn A

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 48h; | 93% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 48h; | 93% |
-
-
131929-60-7
spinosyn A

-
-
131929-68-5
(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-9-ethyl-13-hydroxy-14-methyl-2-(((2R,3R,5S,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-3,3a,5b,6,9,10,11,12,13,14,16a,16b-dodecahydro-1H-as-indaceno[3,2-d][1]oxacyclododecine-7,15(2H,5aH)-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 80 - 90℃; for 24h; | 90% |
| With sulfuric acid In water at 80℃; for 2h; Hydrolysis; | 89% |
| With sulfuric acid | 89% |
| With sulfuric acid In dichloromethane at 80℃; | |
| With sulfuric acid at 80℃; |
-
-
131929-60-7
spinosyn A

-
A
-
56587-30-5
D-forosamine

-
B
-
131929-68-5
(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-9-ethyl-13-hydroxy-14-methyl-2-(((2R,3R,5S,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-3,3a,5b,6,9,10,11,12,13,14,16a,16b-dodecahydro-1H-as-indaceno[3,2-d][1]oxacyclododecine-7,15(2H,5aH)-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid; water at 80℃; for 2h; | A n/a B 89% |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; sulfuric acid In water; dimethyl sulfoxide at 0℃; for 0.166667h; | 86% |
-
-
131929-60-7
spinosyn A

-
-
131929-61-8
N-demethyl-spinosyn A

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In methanol; water for 12h; Reflux; | 81% |
| With iodine; sodium acetate In methanol; water for 12h; Reflux; | 81% |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 3h; | 78% |
-
-
131929-60-7
spinosyn A

-
-
(1aR,1bR,3S,4aS,4bR,7R,8S,12S,15aS,15bR,15cS)-8-(((2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-12-ethyl-7-methyl-3-(((2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2,3,4,4a,7,8,9,10,11,12,15,15a,15b,15c-tetradecahydra-1aH-oxireno[2’,3':4,5]-as-indaceno[3,2-d][1] oxacyclododecine-6,14(1bH,4bH)-dione

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 2.5h; | 76% |
-
-
131929-60-7
spinosyn A

-
-
187168-48-5
4''-ketospinosyn A

| Conditions | Yield |
|---|---|
| With pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate In acetonitrile at 0℃; for 1h; | 63% |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 1h; | 57% |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Stage #1: spinosyn A With mercury(II) trifluoroacetate In tetrahydrofuran; water at 23℃; for 0.5h; Stage #2: With sodium hydroxide; sodium tetrahydroborate Further stages.; | 30.4% |
-
-
131929-60-7
spinosyn A

-
-
149560-97-4
(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-9-ethyl-2,13-dihydroxy-14-methyl-3,3a,5b,6,9,10,11,12,13,14,16a,16b-dodecahydro-1H-as-indaceno[3,2-d][1]oxacyclododecine-7,15(2H,5aH)-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 4.5h; Heating; Yield given; | |
| Multi-step reaction with 2 steps 1: 89 percent / 1 N H2SO4 / H2O / 2 h / 80 °C 2: 66 percent / H2SO4 / methanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: water; sulfuric acid / 2 h / 80 °C 2: water; sulfuric acid / 3 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid 2: sulfuric acid / methanol / Reflux View Scheme |
-
-
131929-60-7
spinosyn A

-
A
-
(1aR,1bR,3S,4aS,4bR,7R,8S,12S,15aS,15bR,15cS)-8-(((2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-12-ethyl-7-methyl-3-(((2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2,3,4,4a,7,8,9,10,11,12,15,15a,15b,15c-tetradecahydra-1aH-oxireno[2’,3':4,5]-as-indaceno[3,2-d][1] oxacyclododecine-6,14(1bH,4bH)-dione

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 23℃; | |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 6h; | A 120 mg B 20 mg |
-
-
131929-60-7
spinosyn A

-
-
187168-79-2
(4''S)-4''-desdimethylamino-4''-hydroxyspinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 63 percent / pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate / acetonitrile / 1 h / 0 °C 2: 99 percent / Li(t-BuO)3AlH / diethyl ether; dioxane / 0.17 h / 0 °C View Scheme |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / aq. H2SO4 / H2O / 24 h / 80 - 90 °C 2: 36 percent / PPTS; BF3*OEt2 / 1,2-dichloro-ethane 3: 42 percent / aq. NH4OAc; Zn / tetrahydrofuran; methanol / 3.5 h / 20 °C View Scheme |
-
-
131929-60-7
spinosyn A

-
-
849420-55-9
C39H61NO10

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 63 percent / pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate / acetonitrile / 1 h / 0 °C 2: 99 percent / Li(t-BuO)3AlH / diethyl ether; dioxane / 0.17 h / 0 °C 3: 91 percent / pyridine; DIPEA / CH2Cl2 / 1 h / 0 °C 4: 39 percent / NaN3 / dimethylformamide / 1 h / 120 °C 5: SnCl2*2H2O / methanol / 20 h / 20 °C View Scheme |
-
-
131929-60-7
spinosyn A

-
-
849420-54-8
(4''R)-4''-desdimethylamino-4''-(azido)spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 63 percent / pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate / acetonitrile / 1 h / 0 °C 2: 99 percent / Li(t-BuO)3AlH / diethyl ether; dioxane / 0.17 h / 0 °C 3: 91 percent / pyridine; DIPEA / CH2Cl2 / 1 h / 0 °C 4: 39 percent / NaN3 / dimethylformamide / 1 h / 120 °C View Scheme |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 63 percent / pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate / acetonitrile / 1 h / 0 °C 2: 99 percent / Li(t-BuO)3AlH / diethyl ether; dioxane / 0.17 h / 0 °C 3: 91 percent / pyridine; DIPEA / CH2Cl2 / 1 h / 0 °C 4: 39 percent / NaN3 / dimethylformamide / 1 h / 120 °C 5: SnCl2*2H2O / methanol / 20 h / 20 °C 6: 7.0 mg / acetonitrile / 2 h / Heating View Scheme |
-
-
131929-60-7
spinosyn A

-
-
849420-51-5
(4''S)-4''-desdimethylamino-4''-(methanesulfonyloxy)spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 63 percent / pyridine; 1-chloromethyl-4-fluorotriethylenediamine tetrafluoroborate / acetonitrile / 1 h / 0 °C 2: 99 percent / Li(t-BuO)3AlH / diethyl ether; dioxane / 0.17 h / 0 °C 3: 91 percent / pyridine; DIPEA / CH2Cl2 / 1 h / 0 °C View Scheme |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / aq. H2SO4 / H2O / 24 h / 80 - 90 °C 2: 36 percent / PPTS; BF3*OEt2 / 1,2-dichloro-ethane View Scheme |
-
-
131929-60-7
spinosyn A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / conc. H2SO4; NBS / dimethylsulfoxide; H2O / 0.17 h / 0 °C 2: 96 percent / NaH / tetrahydrofuran / 0.5 h View Scheme |
Spinosad Chemical Properties
Lepicidin A with CAS NO. of 131929-60-7 is also called for Spinosyn A ; (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-((6-Deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)-13-(((2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-14-methyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-1H-as-indaceno(3,2-d)oxacyclododecin-7,15-dione ; A 83543A ; CCRIS 8937 ; HSDB 7006 ; LY-232105 ; PP-105 ; Spinosad factor A ; UNII-OY0L59V61N ; 1H-as-Indaceno(3,2-d)oxacyclododecin-7,15-dione, 2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-2-((6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)- 13-((5-dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-14-methyl-, (2R-(2R*,3aS*,5aR*,5bS*,9S*,13S*(2R*,5S*,6R*),14R*,16aS*,16bR*))- ; 1H-as-Indaceno(3,2-d)oxacyclododecin-7,15-dione, 2-((6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)-13-(((2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-,(2R,3aS,5aR,5bS,9S,13S, 14R ,16aS, 16bR) - and so on.
Molecular Formula: C41H65NO10
Molecular Weight: 731.96
ACD/LogP: 4.80
ACD/LogD (pH 5.5): 1.99
ACD/LogD (pH 7.4): 3.56
ACD/BCF (pH 5.5): 4.06
ACD/BCF (pH 7.4): 151.13
ACD/KOC (pH 5.5): 15.11
ACD/KOC (pH 7.4): 562.62
H bond acceptors: 11
H bond donors: 0
Freely Rotating Bonds: 9
Heavy Atom Count: 52
Formal Charge: 0
Complexity: 1290
Isotope Atom Count: 0
Defined Atom StereoCenter Count: 16
Undefined Atom StereoCenter Count: 1
Defined Bond StereoCenter Count: 0
Undefined Bond StereoCenter Count: 0
Covalently-Bonded Unit Count: 1
Index of Refraction: 1.54
Molar Refractivity: 196.9 cm3
Molar Volume: 627.2 cm3
Surface Tension: 45.8 dyne/cm
Density: 1.16 g/cm3
Flash Point: 438.5°C
Enthalpy of Vaporization: 116.54 kJ/mol
Boiling Point: 801.5°C at 760 mmHg
Vapour Pressure: 1.04E-25 mmHg at 25°C
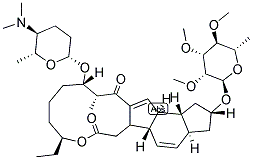
The molecular structure of Lepicidin A with CAS NO. of 131929-60-7:
Spinosad Uses
Lepicidin A with CAS NO. of 131929-60-7 is used as pediculicide.
Spinosad Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD | oral | > 400mg/kg (400mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | National Technical Information Service. Vol. OTS0543604, |
| mouse | LDLo | oral | 500mg/kg (500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0543562, |
| rat | LC50 | inhalation | > 499mg/m3/4H (499mg/m3) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0534564, |
| rat | LD | oral | > 2gm/kg (2000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0543570, |
Spinosad Safety Profile
Moderately toxic by ingestion and inhalation. When heated to decomposition it emits toxic vapors of NOx.
RIDADR: 3077
HazardClass: 9
PackingGroup: III
Hazardous Substances Data: 131929-60-7(Hazardous Substances Data)
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View