-
Name
SULFOACETIC ACID
- EINECS 204-627-2
- CAS No. 123-43-3
- Article Data79
- CAS DataBase
- Density 1.875g/cm3
- Solubility 103.7g/L at 25℃
-
Melting Point
81-88 °C(lit.)
- Formula C2H4 O5 S
- Boiling Point °Cat760mmHg
- Molecular Weight 140.117
- Flash Point °C
- Transport Information
- Appearance
- Safety Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. When heated to decomposition it emits toxic fumes of SOx.
- Risk Codes 34-36/37
-
Molecular Structure
-
Hazard Symbols

- Synonyms Aceticacid, sulfo- (6CI,7CI,8CI,9CI);Sulfoacetic acid;Sulfoethanoic acid;
- PSA 100.05000
- LogP 0.03960
Synthetic route

| Conditions | Yield |
|---|---|
| With nitric acid bei der Oxydation; |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid |
-
-
69406-58-2
chloro-sulfo-acetic acid

-
-
123-43-3
sulfoacetic acid

| Conditions | Yield |
|---|---|
| With sodium amalgam |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With water at 75 - 80℃; bei der elektrolytischen Oxydation an Platin; | |
| With sulfuric acid at 75 - 80℃; bei der elektrolytischen Oxydation an PbO2; |

| Conditions | Yield |
|---|---|
| With chromic acid |
-
-
24523-90-8
(aminoiminomethyl)thioacetic acid ethyl ester

-
-
123-43-3
sulfoacetic acid

| Conditions | Yield |
|---|---|
| With water; chlorine |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II) sulfate |

| Conditions | Yield |
|---|---|
| With tetrachloromethane |

| Conditions | Yield |
|---|---|
| With sodium sulfite |

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With sulfuric acid | |
| With sulfur trioxide; oxygen im UV-Licht; |

| Conditions | Yield |
|---|---|
| With sulfur trioxide; 1,2-dichloro-ethane |

| Conditions | Yield |
|---|---|
| With sulfur trioxide | |
| With chlorosulfonic acid at 140℃; |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid unter Kuehlung und Erhitzen des Reaktionsgemisches dann auf 140grad; |

| Conditions | Yield |
|---|---|
| With nitric acid |

| Conditions | Yield |
|---|---|
| With silver sulfate at 120℃; |

| Conditions | Yield |
|---|---|
| With water; sodium carbonate; sodium sulfite isolierung aus Bariumsalz; |
-
-
89124-45-8
acetic acid ethylester sulfonic acid

-
-
123-43-3
sulfoacetic acid

| Conditions | Yield |
|---|---|
| saponification; |
-
-
64-19-7
acetic acid

-
A
-
123-43-3
sulfoacetic acid

-
B
-
100151-21-1
Acetylsulfat

-
C
-
2480-60-6
diacetyl sulfate

| Conditions | Yield |
|---|---|
| With sulfur trioxide In liquid sulphur dioxide Product distribution; effect of temperature; |


| Conditions | Yield |
|---|---|
| at 40℃; Rate constant; |

-
-
7647-01-0
hydrogenchloride

-
-
37704-67-9
2-ethylamino-thiazol-4-one

-
A
-
625-52-5
N-Ethylurea

-
B
-
123-43-3
sulfoacetic acid

| Conditions | Yield |
|---|---|
| folgend Eindampfen der von Barium befreiten Loesung; |

-
-
108-24-7
acetic anhydride

-
A
-
123-43-3
sulfoacetic acid

-
B
-
503-40-2
methanedisulfonic acid

-
C
-
54322-33-7
methanetrisulfonic acid

| Conditions | Yield |
|---|---|
| je nach den Bedingungen wechselnden Mengen; |

-
-
7647-01-0
hydrogenchloride

-
-
7732-18-5
water

-
-
466-71-7
(16-hydroxy-3β-methoxy-erythrina-1,6-dien-15-yloxysulfonyl)-acetic acid

-
A
-
123-43-3
sulfoacetic acid

-
-
7647-01-0
hydrogenchloride

-
-
7732-18-5
water

-
-
466-79-5
(3β,16-dimethoxy-erythrina-1,6-dien-15-yloxysulfonyl)-acetic acid

-
A
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| at 60℃; unter Ausschluss von Luftfeuchtigkeit und Zersetzen des Reaktionsprodukts mit Eis; bei 4 stuendiger Einw.; |

| Conditions | Yield |
|---|---|
| at 95℃; |

-
-
7722-84-1
dihydrogen peroxide

-
-
68-11-1
mercaptoacetic acid

-
A
-
123-43-3
sulfoacetic acid

-
B
-
505-73-7
disulfanediyldiacetic acid

-
C
-
7664-93-9
sulfuric acid


| Conditions | Yield |
|---|---|
| Heating; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 99% |
-
-
123-43-3
sulfoacetic acid

-
-
1057671-89-2
C15H17NO

-
-
1160847-68-6
N-[3-(3-phenoxyphenyl)propyl](sulfo)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: sulfoacetic acid; C15H17NO With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 2h; Stage #2: In methanol | 85% |
-
-
67-56-1
methanol

-
-
123-43-3
sulfoacetic acid

-
-
220457-32-9, 743422-30-2, 97775-27-4
5α,10β,15α,20β-tetrakis(2-aminophenyl)porphyrinatoiron(III) chloride

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| With isobutyl chlorocarbonate; triethyl amine In dichloromethane sulfoacetic acid added to soln. of isobutyl carbonate and Et3N; stirred at room temp. for 30 min; soln. of Fe compd. in CH2Cl2 added; stirred atroom temp. for 24 h; evapd. to dryness; dissolved in CHCl3; org. layer extd. (aq. NaOH); aq. layer washed (CHCl3); soln. evapd. to dryness; dissolved in EtOH; filtered; filtrate evapd.; purified by chromy. (Cosmosil 75C18-OPN, MeOH-H2O); evapd. to dryness;recrystd. (MeOH-ether); elem. anal.; | 79% |


-
-
123-43-3
sulfoacetic acid

| Conditions | Yield |
|---|---|
| In water Passing a sol. of Pt-complex through a column of an anion-exchange resin (Diaion SA10AOH), passing of an addnl. amount of H2O through the column, addn. of sulfoacetic acid to the eluate.; Concg. filtn., elem. anal.; | 68% |
-
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; ((3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate(V) In N,N-dimethyl-formamide at 20℃; for 16h; | 62% |

| Conditions | Yield |
|---|---|
| Stage #1: C20H39NO4 With triethylamine In tetrahydrofuran Stage #2: sulfoacetic acid With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride In chloroform for 18h; Cooling; Stage #3: With sodium hydrogencarbonate In chloroform | 27.1% |

| Conditions | Yield |
|---|---|
| Stage #1: C22H43NO4 With triethylamine In tetrahydrofuran Stage #2: sulfoacetic acid With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride In chloroform for 18h; Cooling; Stage #3: With sodium hydrogencarbonate In chloroform | 27.1% |

| Conditions | Yield |
|---|---|
| Stage #1: C24H47NO4 With triethylamine In tetrahydrofuran Stage #2: sulfoacetic acid With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride In chloroform for 18h; Cooling; Stage #3: With sodium hydrogencarbonate In chloroform | 27.1% |
-
-
87664-82-2
1,2,3,4-Tetrahydro-5,6-dimethoxy-2-methylisochinolin

-
-
123-43-3
sulfoacetic acid

-
-
87664-92-4
8-Acetyl-1,2,3,4-tetrahydro-5,6-dimethoxy-2-methylisochinolin

| Conditions | Yield |
|---|---|
| 24% |
-
-
123-43-3
sulfoacetic acid

-
-
87664-46-8
1,2-Dimethoxy-5,7,8,9,9a,10-hexahydro-pyrrolo<1,2-b>isochinolin

-
-
87664-49-1
4-Acety-1,2-dimethoxy-5,7,8,9,9a,10-hexahydro-pyrrolo<1,2-b>isochinolin

| Conditions | Yield |
|---|---|
| for 0.0833333h; Heating; | 23% |
-
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; ((3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate(V) In N,N-dimethyl-formamide at 20℃; for 16h; | 20.2% |
-
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 16h; | 17.2% |
-
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 16h; | 7.9% |
-
-
123-43-3
sulfoacetic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 6% |

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; benzene durch anschliessende azeotrope Destillation; |

| Conditions | Yield |
|---|---|
| at 100℃; unter vermindertem Druck; | |
| at 100℃; unter vermindertem Druck; |
Sulfoacetic acid Chemical Properties
Molecular Structure of Sulfoacetic acid (CAS NO.123-43-3):
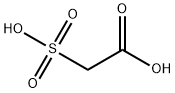
EINECS: 204-627-2
IUPAC Name: 2-Sulfoacetic acid
Molecular Formula: C2H4O5S
Molecular Weight: 140.115160 g/mol
XLogP3-AA: -1.3
H-Bond Donor: 2
H-Bond Acceptor: 5
Canonical SMILES: C(C(=O)O)S(=O)(=O)O
InChI: InChI=1S/C2H4O5S/c3-2(4)1-8(5,6)7/h1H2,(H,3,4)(H,5,6,7)
InChIKey: AGGIJOLULBJGTQ-UHFFFAOYSA-N
Index of Refraction: 1.536
Molar Refractivity: 23.3 cm3
Molar Volume: 74.7 cm3
Surface Tension: 95.2 dyne/cm
Density: 1.875 g/cm3
Melting Point: 81-88 °C(lit.)
Water Solubility: 1.037e+005 mg/L at 25 °C
BRN: 1764390
Sulfoacetic acid Toxicity Data With Reference
| 1. | skn-rbt 20 mg/24H MOD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,1051. | ||
| 2. | eye-rbt 250 µg/24H SEV | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,1051. | ||
| 3. | orl-rat LD50:3160 mg/kg | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. | ||
| 4. | skn-rbt LD50:1570 mg/kg | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. |
Sulfoacetic acid Consensus Reports
Reported in EPA TSCA Inventory.
Sulfoacetic acid Safety Profile
Safety Information of Sulfoacetic acid (CAS NO.123-43-3):
Hazard Codes: C 
Risk Statements: 34-36/37
R34:Causes burns
R36/37:Irritating to eyes and respiratory system
Safety Statements: 26-27-28-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S27:Take off immediately all contaminated clothing
S28:After contact with skin, wash immediately with plenty of soap-suds
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 3261 8/PG 2
WGK Germany: 3
RTECS: AJ4900000
HazardClass: 8
PackingGroup: III
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. When heated to decomposition it emits toxic fumes of SOx.
Sulfoacetic acid Specification
Sulfoacetic acid with CAS registry number of 123-43-3 is also known as 4-04-00-00102 (Beilstein Handbook Reference) ; AI3-28536 ; Acetic acid, sulfo- ; Acetic acid, 2-sulfo- ; HSDB 2706 ; Kyselina sulfooctova ; Kyselina sulfooctova [Czech] ; Sulfoethanoic acid ; Sulphoacetic acid .
Related Products
- Sulfoacetic acid
- 1234-35-1
- 123436-82-8
- 123437-16-1
- 123437-25-2
- 123437-32-1
- 123439-82-7
- 123439-83-8
- 123441-03-2
- 123-44-4
- 123447-62-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View