-
Name
Thiazolidine
- EINECS 208-002-5
- CAS No. 504-78-9
- Article Data30
- CAS DataBase
- Density 1.058 g/cm3
- Solubility
- Melting Point
- Formula C3H7NS
- Boiling Point 164.5 °C at 760 mmHg
- Molecular Weight 89.1613
- Flash Point 56.1 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear colorless liquid
- Safety 45-36/37/39-26
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Thiazolidine
- PSA 37.33000
- LogP 0.60910
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphate buffer In water at 25℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; for 0.5h; Mechanism; var. buffer systems; var. aldehydes; also in the presence of ethanol; |

-
-
504-78-9
1,3-thiazolidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
148312-55-4
tert-butyl thiazolidine-3-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water at 20℃; for 20h; | 100% |
| In tetrahydrofuran; water at 20℃; for 20h; Schlenk technique; | 99% |
| With dmap In acetonitrile for 3h; Ambient temperature; | 89% |
| In 1,4-dioxane; water | 86.2% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; N,N-dimethyl-formamide a) -5 deg C, 2 h, b) RT, 20 h; | 97% |
| With potassium carbonate In hexane; ethyl acetate; N,N-dimethyl-formamide | 97.2% |
-
-
504-78-9
1,3-thiazolidine

-
-
841302-34-9
(S)-[1-(3-hydroxyadamantan-1-yl)-2-oxo-2-pyrrolidin-1-ylethyl]-carbamic acid tert-butyl ester

-
-
841302-35-0
(S)-[1-(3-hydroxyadamantan-1-yl)-2-oxo-2-thiazolidin-3-ylethyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In DMF (N,N-dimethyl-formamide) at 0℃; for 22h; | 95.7% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.75h; | 95% |
-
-
504-78-9
1,3-thiazolidine

-
-
143815-60-5
2-chloro-5-cyano-6-ethoxy-4-phenylpyridine-3-carboxaldehyde

-
-
143815-68-3
2-Ethoxy-5-formyl-4-phenyl-6-thiazolidin-3-yl-nicotinonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 0.166667h; Heating; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-thiazolidine; N-Boc pyroglutamic acid-Wang resin In tetrahydrofuran Addition; Stage #2: trifluoroacetic acid In water for 2.5h; Hydrolysis; | 93% |

-
-
504-78-9
1,3-thiazolidine

-
-
336870-02-1
(S)-tert-butoxycarbonylamino-(trans-4-hydroxy-cyclohexyl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-tert-butoxycarbonylamino-(trans-4-hydroxy-cyclohexyl)-acetic acid With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In Isopropyl acetate at 0℃; for 2h; Stage #2: 1,3-thiazolidine In Isopropyl acetate at 0 - 20℃; for 18h; | 93% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; for 12h; |
-
-
504-78-9
1,3-thiazolidine

-
-
682349-94-6
3-((2R,3S,4R,5R,6R)-3,4,5-Triacetoxy-6-acetoxymethyl-tetrahydro-pyran-2-yl)-propionic acid pentafluorophenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 92% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.75h; | 92% |
| With triethylamine In dichloromethane |
-
-
504-78-9
1,3-thiazolidine

-
-
1196-67-4, 21878-52-4, 21866-70-6
(E)-β-methylcinnamaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: (E)-β-methylcinnamaldehyde With 1,4-diaza-bicyclo[2.2.2]octane; 1H-imidazole; BF4(1-)*C21H22N3O(1+); 1,1'-biphenyl-2,2'-diyl hydrogen phosphate In toluene at 40℃; for 14h; Molecular sieve; Sealed tube; Inert atmosphere; Stage #2: 1,3-thiazolidine In toluene at 20℃; for 10h; Molecular sieve; Sealed tube; Inert atmosphere; enantioselective reaction; | 92% |
-
-
504-78-9
1,3-thiazolidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
102373-23-9
1-aminocyclohexanecarboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: di-tert-butyl dicarbonate; 1-aminocyclohexanecarboxylic acid benzyl ester With dmap In dichloromethane at 20℃; for 0.666667h; Stage #2: 1,3-thiazolidine With triethylamine In dichloromethane at 20℃; for 19h; | 91% |


| Conditions | Yield |
|---|---|
| With sodium carbonate | 90% |
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; for 4h; |
-
-
504-78-9
1,3-thiazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1,4-dioxane at 100℃; for 16h; | 90% |
-
-
504-78-9
1,3-thiazolidine

-
-
59969-65-2
(2S,3R)-3-benzyloxycarbonylamino-2-hydroxy-4-phenyl-butanoic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 4 - 20℃; | 90% |
-
-
504-78-9
1,3-thiazolidine

-
-
114346-31-5
(S)-3-(4-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)phenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid

-
-
937207-80-2
C32H35N3O5S

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In tetrahydrofuran at 0 - 20℃; for 8h; | 90% |
-
-
504-78-9
1,3-thiazolidine

-
-
102195-80-2
(S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate

-
-
401564-36-1
(2S)-4-oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 0 - 30℃; for 2.5h; Large scale; | 90% |
-
-
504-78-9
1,3-thiazolidine

-
-
1174039-19-0
(R)-7-[3-tertbutoxycarbonylamino-4-(2,4,5-tri-fluorophenyl)butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid

-
-
1174039-38-3
(R)-[3-oxo-3-[1-(thiazolidine-3-carbonyl)-3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl]-1-(2,4,5-trifluoro-benzyl)-propyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride; triethylamine In dichloromethane at 20℃; for 2h; | 89% |
-
-
504-78-9
1,3-thiazolidine

-
-
75-15-0
carbon disulfide

-
-
31581-11-0
2,3-bis(chloromethyl)-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-thiazolidine; carbon disulfide In acetonitrile at 20℃; for 0.5h; Stage #2: 2,3-bis(chloromethyl)-1,4-naphthoquinone In acetonitrile at 20℃; for 48h; | 88.8% |

-
-
504-78-9
1,3-thiazolidine

-
-
88329-34-4, 122230-50-6
1-Naphthylacetyl-thioproline

-
-
122230-45-9
(1-Naphthyl)acetyl-Thiopro-thiazolidine

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide | 88% |
-
-
504-78-9
1,3-thiazolidine

-
-
115962-35-1, 144069-68-1, 78879-20-6
(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinole-3-carboxylic acid

-
-
460086-07-1
3-(thiazolidine-3-carbonyl)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane; N,N-dimethyl-formamide at 20℃; for 1.5h; | 88% |
-
-
504-78-9
1,3-thiazolidine

-
-
18496-54-3
phenylbutyric acid chloride

-
-
127401-86-9
N-(4-phenylbutanoyl)thiazolidine

| Conditions | Yield |
|---|---|
| With sodium chloride; triethylamine In dichloromethane | 88% |
-
-
504-78-9
1,3-thiazolidine

-
-
78461-93-5
S-6-bromohexanoic acid N-α-methylbenzylamide

-
-
78461-95-7
S-6-(3-thiazolidinyl)hexanoic acid N-α-methylbenzylamide

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol for 6h; Heating; | 87% |
-
-
504-78-9
1,3-thiazolidine

-
-
78461-92-4
R-6-bromohexanoic acid N-α-methylbenzylamide

-
-
78461-94-6
R-6-(3-thiazolidinyl)hexanoic acid N-α-methylbenzylamide

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol for 6h; Heating; | 87% |
-
-
504-78-9
1,3-thiazolidine

-
-
898273-26-2
(2S,4S)-1-tert-butoxycarbonyl-4-ethoxycarbonyl-2-pyrrolidinecarboxylic acid

-
-
1019637-84-3
1-tert-butyl 3-ethyl (3S,5S)-5-(1,3-thiazolidin-3-ylcarbonyl)-1,3-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 19h; | 86% |

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In tetrahydrofuran at 0 - 20℃; for 4h; | 86% |
-
-
504-78-9
1,3-thiazolidine

-
-
84348-37-8
N-tert-butoxycarbonyl-4-oxo-L-proline

-
-
401564-36-1
(2S)-4-oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 2 - 7℃; for 2h; Large scale; | 86% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With pivaloyl chloride; N-ethyl-N,N-diisopropylamine In ethyl acetate at 10℃; for 0.5h; Large scale; Stage #2: 1,3-thiazolidine In ethyl acetate at 0 - 10℃; for 1h; Reagent/catalyst; Temperature; Solvent; Large scale; | 86% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With dicyclohexyl-carbodiimide In toluene at -10 - -5℃; Stage #2: 1,3-thiazolidine With dmap In toluene at -6 - 5℃; for 1.33333h; | 81.7% |
| With dmap; dicyclohexyl-carbodiimide In toluene at -6 - 5℃; for 1.33333h; | 81.7% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In toluene at 0℃; for 3h; Large scale; Stage #2: 1,3-thiazolidine With dmap at 0℃; for 2h; Reagent/catalyst; Large scale; | 50 kg |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 18h; | 85% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol | 82% |
-
-
504-78-9
1,3-thiazolidine

-
-
867212-43-9
(S)-{trans-4-[(3-amino-pyrazine-2-carbonyl)-amino]-cyclohexyl}-tert-butoxycarbonylamino-acetic acid

-
-
867212-44-0
(S)-(1-{trans-4-[(3-amino-pyrazine-2-carbonyl)-amino]-cyclohexyl}-2-oxo-2-thiazolidin-3-yl-ethyl)-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 4h; | 82% |
Thiazolidine Specification
The Thiazolidine, with the CAS registry number 504-78-9 and EINECS registry number 208-002-5, has the systematic name and IUPAC name of 1,3-thiazolidine. It is a kind of clear colorless liquid, and belongs to the following product categories: Heterocyclic Compounds; Building Blocks; Heterocyclic Building Blocks; Thiazolines/Thiazolidines. And the molecular formula of the chemical is C3H7NS.
The characteristics of Thiazolidine are as followings: (1)ACD/LogP: -0.12; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.03; (4)ACD/LogD (pH 7.4): -1.57; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 28.54 Å2; (13)Index of Refraction: 1.513; (14)Molar Refractivity: 25.34 cm3; (15)Molar Volume: 84.2 cm3; (16)Polarizability: 10.04×10-24cm3; (17)Surface Tension: 37.5 dyne/cm; (18)Density: 1.058 g/cm3; (19)Flash Point: 56.1 °C; (20)Enthalpy of Vaporization: 40.1 kJ/mol; (21)Boiling Point: 164.5 °C at 760 mmHg; (22)Vapour Pressure: 1.96 mmHg at 25°C.
It may be synthesized by a condensation reaction between a thiol and an aldehyde or ketone. The reaction is reversible. Therefore,many thiazolidines are labile towards hydrolysis in aqueous solution. Hydrolysis of the thiazolidine generates the thiol and aldehyde or ketone from which it was synthesized.
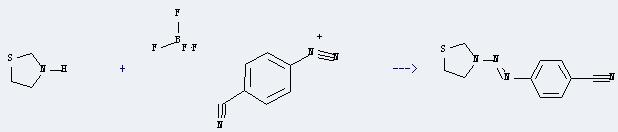
Uses of Thiazolidine: It can react with 4-cyano-benzenediazonium; tetrafluoroborate to produce 3-(p-cyanophenylazo)-1,3-thiazolidine. This reaction will need the menstruum acetone and H2O. The reaction time is 0.5 hours with temperature of 0°C, and the yield is about 37%.
You should be cautious while dealing with this chemical. It may cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: S1CCNC1
(2)InChI: InChI=1/C3H7NS/c1-2-5-3-4-1/h4H,1-3H2
(3)InChIKey: OGYGFUAIIOPWQD-UHFFFAOYAA
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | Farmaco, Edizione Scientifica. Vol. 36, Pg. 740, 1981. |
Related Products
- Thiazolidine
- Thiazolidine, 2-(o-methoxyphenyl)-
- Thiazolidine, 2-(p-nitrophenyl)-
- Thiazolidine, 2-phenyl-
- Thiazolidine,2-(4-chlorophenyl)-5-methyl-
- Thiazolidine,2-(4-ethylphenyl)-
- Thiazolidine,2-(4-fluorophenyl)-3-methyl-
- Thiazolidine,2-(4-methoxyphenyl)-
- Thiazolidine,2,5-dimethyl-2-phenyl-
- Thiazolidine,2-[(2-methoxyphenoxy)methyl]-
- 50479-11-3
- 50480-29-0
- 50481-48-6
- 5048-25-9
- 50483-77-7
- 50483-79-9
- 5048-50-0
- 504-85-8
- 50-48-6
- 50487-70-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View