-
Name
Tributyrin
- EINECS 200-451-5
- CAS No. 60-01-5
- Article Data24
- CAS DataBase
- Density 1.054 g/cm3
- Solubility Not miscible in water.
- Melting Point -75 °C
- Formula C15H26O6
- Boiling Point 307.499 °C at 760 mmHg
- Molecular Weight 302.368
- Flash Point 143.415 °C
- Transport Information
- Appearance colourless liquid
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Butyrin,tri- (6CI,8CI);Butyryl triglyceride;Glycerin tributyrate;Glyceroltributanoate;Glycerol tributyrate;Glyceroltributyrin;Glyceryl tributanoate;Glyceryl tributyrate;NSC 661583;Tri-n-butyrin;Tributanoin;Tributin;Tributyrin;Tributyroin;Tributyryl glyceride;Tributyrylglycerol;
- PSA 78.90000
- LogP 2.38490
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphoric acid; aminosulfonic acid at 100℃; for 12h; Catalytic behavior; Reagent/catalyst; Temperature; Green chemistry; | 96.17% |
| With sodium hydrogen sulfate at 150℃; Reagent/catalyst; Large scale; | 96.49% |
| With sulfonated charcoal In benzene for 7h; Heating; | 94% |

| Conditions | Yield |
|---|---|
| With SBA-15-sulfonic acid mesoporous molecular sieve; ZSM-5 acid zeolite at 100℃; Temperature; Green chemistry; | 95.3% |


| Conditions | Yield |
|---|---|
| With xylene |

| Conditions | Yield |
|---|---|
| With camphoric acid | |
| With sulfuric acid at 204℃; |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride at 200℃; |

| Conditions | Yield |
|---|---|
| unter staendiger Entfernung des entstehenden Wassers; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pyridine; water; glycerol | 29.6 g (98%) |
-
-
56-81-5
glycerol

-
-
107-92-6
butyric acid

-
A
-
60-01-5
tributyrin

-
B
-
557-25-5
1-monobutyrin

-
C
-
30403-46-4, 96739-06-9, 101469-20-9, 24814-35-5
β-dibutyrin

-
D
-
70916-53-9
2-monobutyroylglycerol

-
E
-
17364-00-0
1,3-dibutanoyloxy-2-propanol

| Conditions | Yield |
|---|---|
| at 115℃; for 24h; |

-
-
56-81-5
glycerol

-
-
107-92-6
butyric acid

-
A
-
60-01-5
tributyrin

-
B
-
30403-46-4, 96739-06-9, 101469-20-9, 24814-35-5
β-dibutyrin

-
C
-
17364-00-0
1,3-dibutanoyloxy-2-propanol

| Conditions | Yield |
|---|---|
| With sulphonated hydrothermal carbon at 115℃; for 24h; Catalytic behavior; chemoselective reaction; |


| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 3h; Time; Cooling with ice; | 39.9 g |

| Conditions | Yield |
|---|---|
| With graphite oxide at 80℃; for 3h; regioselective reaction; | 97 %Chromat. |

| Conditions | Yield |
|---|---|
| With strontium hydroxide; dihexyl ether at 60℃; Reagent/catalyst; Concentration; Time; | 98% |
| With carbon-based sulfonated solid acid prepared at 150 °C at 80℃; for 8h; Catalytic behavior; Kinetics; Reagent/catalyst; | 97.2% |
| With Et3N-grafted carbon nanotubes at 60℃; for 8h; Inert atmosphere; |
-
-
60-01-5
tributyrin

-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
A
-
1445-91-6
(S)-1-phenylethanol

-
B
-
89378-61-0
(R)-1-phenylethyl butyrate

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 40℃; for 6h; Kinetics; Temperature; Resolution of racemate; Enzymatic reaction; enantioselective reaction; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With ammonia at 220℃; for 40h; Autoclave; | 94% |

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); hydrogen; bis(trifluoromethanesulfonyl)amide; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 130℃; under 45004.5 Torr; for 18h; Autoclave; | 90% |

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) at 150℃; for 24h; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); hydrogen; bis(trifluoromethanesulfonyl)amide; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 130℃; under 45004.5 Torr; for 18h; Autoclave; | 89% |
-
-
60-01-5
tributyrin

-
-
86227-47-6
eicosapentaenoic acid ethyl ester

-
-
99660-94-3
triglyceride of eicosapentaenoic acid

| Conditions | Yield |
|---|---|
| With immobilized lipase from Candida Antarctica at 65℃; under 0.01 - 0.1 Torr; for 72h; | 86% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)amino]ruthenium(II); hydrogen In toluene at 100℃; under 30003 Torr; for 18h; Autoclave; Inert atmosphere; Glovebox; | 84% |
| With 5 wt% Re/TiO2; hydrogen In neat (no solvent) at 220℃; under 37503.8 Torr; for 24h; Autoclave; | 83% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 140h; Ambient temperature; yeast lipase catalyzed transesterification; | 82% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 150h; Ambient temperature; yeast lipase catalyzed transesterification; | 82% |
-
-
60-01-5
tributyrin

-
-
81926-94-5
all-(Z)-ethyl 4,7,10,13,16,19-docosahexaenoate

-
-
124596-98-1
triglyceride of docosahexaenoic acid

| Conditions | Yield |
|---|---|
| With immobilized lipase from Candida Antarctica at 65℃; under 0.01 - 0.1 Torr; for 72h; | 81% |
-
-
60-01-5
tributyrin

-
-
17272-83-2
N1-benzyl-benzene-1,4-diamine

-
-
1194828-79-9
N1-benzyl-N4-butylbenzene-1,4-diamine

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); hydrogen; bis(trifluoromethanesulfonyl)amide; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 130℃; under 45004.5 Torr; for 18h; Autoclave; | 80% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 128h; Ambient temperature; yeast lipase catalyzed transesterification; | 79% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 36h; Ambient temperature; yeast lipase catalyzed transesterification; | 79% |

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); hydrogen; bis(trifluoromethanesulfonyl)amide; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 130℃; under 45004.5 Torr; for 18h; Autoclave; | 78% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 52h; Ambient temperature; yeast lipase catalyzed transesterification; | 76% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 40h; Ambient temperature; yeast lipase catalyzed transesterification; | 75% |

| Conditions | Yield |
|---|---|
| With phosphate buffer for 50h; Ambient temperature; yeast lipase catalyzed transesterification; | 70% |

-
-
60-01-5
tributyrin

-
-
103253-60-7, 121522-26-7, 121522-27-8, 6999-19-5
4-trimethylsilyl-3-butyn-2-ol

| Conditions | Yield |
|---|---|
| 70% |


| Conditions | Yield |
|---|---|
| With hafnium(IV) trifluoromethanesulfonate at 120℃; for 24h; Sealed tube; | 48% |
-
-
60-01-5
tributyrin

-
-
17364-00-0
1,3-dibutanoyloxy-2-propanol

| Conditions | Yield |
|---|---|
| bei der Spaltung durch Pankreaslipase; |

| Conditions | Yield |
|---|---|
| In water at 35 - 37℃; for 140h; Candida cylindracea lipase immobilized on Amberlite IRA-938, pH 7.3; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| In water at 35 - 37℃; for 140h; Candida cylindracea lipase immobilized on Amberlite IRA-938, pH 7.3; Yield given; |

| Conditions | Yield |
|---|---|
| In water at 35 - 37℃; for 140h; Candida cylindracea lipase immobilized on Amberlite IRA-938, pH 7.3; Yield given. Yields of byproduct given; |

Tributyrin Consensus Reports
Tributyrin Specification
The Tributyrin, with the CAS registry number 60-01-5, is also known as Butyrin, tri-. It belongs to the product categories of Functional Materials; Plasticizer; Polyalcohol Ethers, Esters (Plasticizer). Its EINECS registry number is 200-451-5. This chemical's molecular formula is C15H26O6 and molecular weight is 302.36. Its IUPAC name is called 2,3-di(butanoyloxy)propyl butanoate. When you are using this chemical, please be cautious about it. You must avoid contact with skin and eyes. Tributyrin is also used in microbiological laboratories to identify the bacterium Moraxella catarrhalis. Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells.
Physical properties of Tributyrin: (1)ACD/LogP: 3.27; (2)ACD/LogD (pH 5.5): 3.273; (3)ACD/LogD (pH 7.4): 3.273; (4)ACD/BCF (pH 5.5): 180.955; (5)ACD/BCF (pH 7.4): 180.955; (6)ACD/KOC (pH 5.5): 1437.395; (7)ACD/KOC (pH 7.4): 1437.395; (8)#H bond acceptors: 6; (9)#Freely Rotating Bonds: 14; (10)Index of Refraction: 1.448; (11)Molar Refractivity: 76.825 cm3; (12)Molar Volume: 286.96 cm3; (13)Surface Tension: 35.51 dyne/cm; (14)Density: 1.054 g/cm3; (15)Flash Point: 143.415 °C; (16)Enthalpy of Vaporization: 54.81 kJ/mol; (17)Boiling Point: 307.499 °C at 760 mmHg; (18)Vapour Pressure: 0.001 mmHg at 25°C.
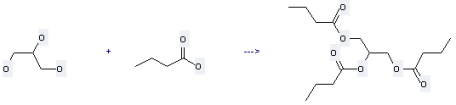
Preparation of Tributyrin: this chemical can be prepared by butyric acid and propane-1,2,3-triol. This reaction will need reagent aluminium sulfate.
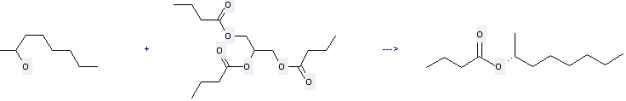
Uses of Tributyrin: it can be used to produce (R)-2-octyl butyrate at ambient temperature. This reaction will need reagent 0.1 M phosphate buffer with reaction time of 140 hours. The yield is about 82%.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CCCC(=O)OCC(COC(=O)CCC)OC(=O)CCC
(2)InChI: InChI=1S/C15H26O6/c1-4-7-13(16)19-10-12(21-15(18)9-6-3)11-20-14(17)8-5-2/h12H,4-11H2,1-3H3
(3)InChIKey: UYXTWWCETRIEDR-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 320mg/kg (320mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Acta Physiologica Scandinavica. Vol. 40, Pg. 338, 1957. |
| mouse | LD50 | oral | 12800mg/kg (12800mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LC | inhalation | > 75ppm/6H (75ppm) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LD50 | oral | 3200mg/kg (3200mg/kg) | Kodak Company Reports. Vol. 21MAY1971, |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View