-
Name
Triethylsilane
- EINECS 210-535-3
- CAS No. 617-86-7
- Article Data105
- CAS DataBase
- Density 0.728g/mLat 25°C(lit.)
- Solubility Miscible with water.
- Melting Point -157 °C
- Formula C6H16Si
- Boiling Point 107.499 °C at 760 mmHg
- Molecular Weight 116.279
- Flash Point 25°F
- Transport Information UN 1993 3/PG 2
- Appearance Clear liquid
- Safety 9-16-29-33-37/39-26
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms Triethylsilyl hydride;
- PSA 0.00000
- LogP 2.27320
Synthetic route

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium tetrahydroborate; ethyl bromide; tetraoctyl ammonium bromide In benzene-d6 at 20℃; for 24h; Reagent/catalyst; Solvent; | 96% |
-
-
13399-93-4
3-(trimethylsilyl)propane-1-thiol

-
-
4149-29-5
bis(triethylsilyl)mercury

-
A
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| In benzene Heating; | A n/a B 89.5% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In acetonitrile at 20℃; for 0.25h; Inert atmosphere; | 88% |
| With lithium hydride In tetrahydrofuran at 20 - 60℃; Reagent/catalyst; Solvent; | 82% |
| Stage #1: triethylsilyl chloride With magnesium In tetrahydrofuran at 75℃; for 12h; Inert atmosphere; Stage #2: Acidic conditions; | 80% |
-
-
7538-45-6
2-mercaptoethyl(trimethoxysilane)

-
-
4149-29-5
bis(triethylsilyl)mercury

-
A
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| In benzene Heating; | A n/a B 87.3% |

-
-
30817-94-8
(mercaptomethyl)trimethoxysilane

-
-
4149-29-5
bis(triethylsilyl)mercury

-
A
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| In benzene at 50℃; for 0.25h; | A n/a B 80% |

-
-
109-65-9
1-bromo-butane

-
-
92465-73-1
triethylsilylcalcium chloride

-
A
-
617-86-7
triethylsilane

-
B
-
994-96-7
butyltriethylsilane

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | A 1.1% B 26% |

-
-
108-86-1
bromobenzene

-
-
92465-73-1
triethylsilylcalcium chloride

-
A
-
617-86-7
triethylsilane

-
B
-
2987-77-1
triethylphenylsilane

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | A 3% B 24% |

-
-
462-06-6
fluorobenzene

-
-
92465-73-1
triethylsilylcalcium chloride

-
A
-
617-86-7
triethylsilane

-
B
-
2987-77-1
triethylphenylsilane

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | A 15% B n/a |

-
-
92465-73-1
triethylsilylcalcium chloride

-
-
108-90-7
chlorobenzene

-
A
-
617-86-7
triethylsilane

-
B
-
2987-77-1
triethylphenylsilane

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | A 10% B 5% |


| Conditions | Yield |
|---|---|
| With sodium |

| Conditions | Yield |
|---|---|
| With diethylaluminium hydride | |
| Multi-step reaction with 2 steps 1: I2; AlI3 View Scheme |

| Conditions | Yield |
|---|---|
| With sodium | |
| With Na In not given |

| Conditions | Yield |
|---|---|
| With triethylaluminum |

| Conditions | Yield |
|---|---|
| With diethyl ether; trichlorosilane | |
| With trichlorosilane In diethyl ether |

| Conditions | Yield |
|---|---|
| With diethyl ether; trichlorosilane |

| Conditions | Yield |
|---|---|
| With sulfur trioxide; zirconium(IV) oxide at 99.9℃; under 30 Torr; for 0.583333h; Product distribution; other alkylsilanes, var. temp., var. pretreatment of catalyst; |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; ytterbium In tetrahydrofuran Yield given; |
-
-
925-90-6
ethylmagnesium bromide

-
-
101519-12-4
sodiumtris(benzene-1,2 diolato)silicate

-
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride Yield given. Multistep reaction; |
-
-
101631-06-5
triethylsilyl hydrotrioxide

-
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| In dichloromethane at -60℃; for 2.5h; |
-
-
994-30-9
triethylsilyl chloride

-
-
71-43-2
benzene

-
A
-
617-86-7
triethylsilane

-
B
-
2987-77-1
triethylphenylsilane

| Conditions | Yield |
|---|---|
| With calcium 1) 900 deg C, 0.005 Torr, 2) 1 h, r.t., 0.005 Torr; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| cationic dirhodium(II) complex at 50℃; for 24h; | 100% |
| With Rh/AlO(OH) In toluene at 25℃; for 24h; Inert atmosphere; | 90% |
| dirhodium(II) tetrakis(perfluorobutyrate) In dichloromethane for 3h; Product distribution; Ambient temperature; var. alcohols; rel. reactivities for triethylsilane alcoholysis; var. catalysts; | 86% |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; water In ethyl acetate | 100% |
| With water at 20℃; for 0.25h; | 99% |
| With water In tetrahydrofuran at 25℃; for 0.0166667h; Catalytic behavior; | 99% |
-
-
617-86-7
triethylsilane

-
-
80-62-6
methacrylic acid methyl ester

-
-
18002-64-7
β-triethylsilanyl-isobutyric acid methyl ester

| Conditions | Yield |
|---|---|
| bis(benzonitrile)dichloroplatinum(II); triethylphosphine for 24h; | 100% |
| With platinum on activated charcoal | |
| With dihydrogen hexachloroplatinate |

| Conditions | Yield |
|---|---|
| With indium(III) bromide In dichloromethane-d2 at 20℃; | 100% |
| With palladium diacetate In benzene-d6 for 4h; dehydrocoupling reaction; Heating; | 95% |
| With zinc(II) chloride In N,N-dimethyl-formamide at 120℃; for 25h; | 85% |

| Conditions | Yield |
|---|---|
| cerium(IV) oxide; gold In toluene at 70℃; for 8h; | 100% |
| With tris(pentafluorophenyl)borate In dichloromethane at 20℃; for 12h; | 96% |
| With dimanganese decacarbonyl; bis(3,5-bis(trifluoromethyl)phenyl)(2’,4’,6’-triisopropyl-3,6-dimethoxy-[1,1’-biphenyl]-2-yl)phosphine at 120℃; for 24h; Sealed tube; | 83% |

| Conditions | Yield |
|---|---|
| at 90℃; for 5h; Inert atmosphere; | 100% |
| at 90℃; for 5h; Inert atmosphere; | 100% |
| With C26H31F5NZn(1+)*C24BF20(1-) In dichloromethane-d2 at 20℃; for 28h; Reagent/catalyst; Inert atmosphere; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| cerium(IV) oxide; gold In toluene at 70℃; for 8h; | 100% |
| With C16H25AlBN6(1+)*C19H3BF15(1-) In chloroform-d1 at 75℃; for 0.5h; | 99% |
| With (1,3-dimesityl-5-methyl-6-oxo-6H-pyrimidin-2-ylidene-4-olate)-copper(I) lithium(THF)2; sodium t-butanolate In tetrahydrofuran at 65℃; for 2h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With (1,3-dimesityl-5-methyl-6-oxo-6H-pyrimidin-2-ylidene-4-olate)-copper(I) lithium(THF)2; sodium t-butanolate In tetrahydrofuran at 65℃; for 1h; Reagent/catalyst; Temperature; Time; Schlenk technique; Inert atmosphere; | 100% |
| With C42H43BN3P2(1+)*C18HBF15(1-) In chloroform at 40℃; for 2h; Reagent/catalyst; Temperature; Schlenk technique; Glovebox; | 99% |
| Stage #1: acetophenone With C8H10BiCl2N; C4H6AgN2(1+)*C24H12BCl8(1-) In dichloromethane-d2 at 25℃; Stage #2: triethylsilane In dichloromethane at 25℃; | 99% |
-
-
617-86-7
triethylsilane

-
-
821-10-3
1,4-Dichloro-2-butyne

- 1,4-dichloro 2-triethylsilylbut-2-ene
-
108930-44-5
1,4-dichloro 2-triethylsilylbut-2-ene

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In tetrahydrofuran at 85℃; for 4h; | 100% |
| With platinum(IV) oxide In toluene at 100℃; for 24h; diastereoselective reaction; | 75% |
| dihydrogen hexachloroplatinate | |
| With dihydrogen hexachloroplatinate at 170℃; for 1h; |

| Conditions | Yield |
|---|---|
| {Rh2(OAc)3[(R)-PhCH2SCH(Me)C(O)OEt]2}BF4 In dichloromethane at 50℃; for 24h; | 100% |
| With C23H26IrN2(1+)*C32H12BF24(1-) In 1,2-dichloro-ethane at 20℃; for 0.0166667h; Catalytic behavior; Schlenk technique; Inert atmosphere; | 100% |
| With Pd/XC-72-700-Ar at 25℃; for 0.03h; | 99% |

| Conditions | Yield |
|---|---|
| nickel at 100℃; for 1h; | 100% |
| With [(POCOP)Ir(H)(acetone)]+[B(C6F5)4]- In dichloromethane-d2 at 22℃; for 3h; | 100 %Spectr. |
-
-
617-86-7
triethylsilane

-
-
622-97-9
1-ethenyl-4-methylbenzene

-
-
75476-54-9
(E)-1-(p-methylphenyl)-2-(triethylsilyl)ethylene

| Conditions | Yield |
|---|---|
| dodecacarbonyl-triangulo-triruthenium In benzene at 80℃; for 5h; | 100% |
| With dimanganese decacarbonyl; N,N-bis(diphenylphosphino)isopropylamine at 140℃; for 24h; Sealed tube; | 82% |
| With norborn-2-ene; chloro(1,5-cyclooctadiene)rhodium(I) dimer; triphenylphosphine In tetrahydrofuran at 100℃; for 2h; Schlenk technique; Inert atmosphere; Sealed tube; | 82% |
-
-
617-86-7
triethylsilane

-
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
57137-74-3
(E)-4-methyl-2-(triethylsilyloxy)pent-2-ene

| Conditions | Yield |
|---|---|
| RhCl(PPh3)3 at 40℃; | 100% |
-
-
617-86-7
triethylsilane

-
-
4526-07-2
1,4-bis(trimethylsilyl)-1,3-butadiyne

-
-
103716-77-4
(E)-2-triethylsilyl-1,4-bis(trimethylsilyl)-1-buten-3-yne

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine)platinum at 100℃; for 1h; | 100% |
-
-
617-86-7
triethylsilane

-
-
4526-07-2
1,4-bis(trimethylsilyl)-1,3-butadiyne

-
-
103716-76-3
1,3-bis(triethylsilyl)-1,4-bis(trimethylsilyl)-1,2-butadiene

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate In isopropyl alcohol at 80℃; for 0.5h; | 100% |
| With dihydrogen hexachloroplatinate In isopropyl alcohol at 80℃; for 0.5h; | 100% |
-
-
617-86-7
triethylsilane

-
-
123239-66-7
(3-methoxynaphthalen-2-yl)phenylmethanol

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; | 100% |
-
-
617-86-7
triethylsilane

-
-
103716-81-0
(E)-2-dimethylphenylsilyl-1,4-bis(trimethylsilyl)-1-buten-3-yne

-
-
103716-84-3
3-dimethylphenylsilyl-1-triethylsilyl-1,4-bis(trimethylsilyl)-1,2-butadiene

| Conditions | Yield |
|---|---|
| With RhCl(PPh3)3 at 100℃; for 13h; | 100% |
| dihydrogen hexachloroplatinate | 93% |
-
-
617-86-7
triethylsilane

-
-
104106-61-8
4aβ,8-dimethyl-4a,5,6,8aα-tetrahydro-2<1H>-naphthalenone

-
-
104504-88-3
7-triethylsilyloxy-1,4aβ-dimethyl-3,4,4a,5,8,8aα-hexahydronaphthalene

| Conditions | Yield |
|---|---|
| Wilkinson's catalyst for 6h; Ambient temperature; | 100% |
-
-
617-86-7
triethylsilane

-
-
151545-05-0
C72H140Si14

| Conditions | Yield |
|---|---|
| In toluene at 70℃; | 100% |
| In tetrahydrofuran for 10h; Heating; | 77% |
| In toluene at 60℃; Rate constant; Mechanism; Kinetics; energy data: Ea, ΔH(excit.), SDS(excit.); var. temp.; | |
| In toluene at 81.3℃; Rate constant; Kinetics; Thermodynamic data; Ea, ΔH(excit.), SDS(excit.); var. temp.; |

| Conditions | Yield |
|---|---|
| platinum(II) chloride at 24.9℃; for 96h; | 100% |
| With fluorotris(pentafluorophenyl)phosphonium tetrakis(pentafluorophenyl)borate In dichloromethane at 20℃; for 1h; Inert atmosphere; | 99% |
| With tris(pentafluorophenyl)borate In dichloromethane at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| carbonylhydridetris(triphenylphosphine)rhodium(I) at 20℃; for 72h; magnetic field 40 Oe; | 100% |
| With dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl; platinum(II) chloride In tetrahydrofuran at 60℃; for 1.25h; | 98% |
| With sodium iodide In tetrahydrofuran at 20℃; for 1h; Catalytic behavior; stereoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| In (2)H8-toluene at 120℃; for 30h; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In (2)H8-toluene at 120℃; for 30h; | A 100% B n/a |

-
-
617-86-7
triethylsilane

-
-
218937-15-6
2α,9α,10β,13α-tetraacetoxy-taxa-4(20),11-dien-5α-ol

-
-
325691-23-4
2α,9α,10β,13α-tetraacetoxy-5α-triethylsilyloxytaxa-4(20),11-diene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 2h; | 100% |
-
-
617-86-7
triethylsilane

-
-
51060-11-8
2,2,4-trimethyl-hept-6-yn-3-one

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate In toluene at 0℃; for 1h; | 100% |
-
-
617-86-7
triethylsilane

-
-
552890-39-8
Acetic acid (2R,3S,5R,6S)-6-allyl-5-benzyloxy-2-phenylsulfanylethynyl-tetrahydro-pyran-3-yl ester

-
-
617673-36-6
Acetic acid (2R,3S,5R,6S)-6-allyl-5-benzyloxy-2-((Z)-2-phenylsulfanyl-2-triethylsilanyl-vinyl)-tetrahydro-pyran-3-yl ester

| Conditions | Yield |
|---|---|
| biscobalthexacarbonyl-2-methyl-but-3-yn-2-ol In 1,2-dichloro-ethane at 60℃; for 1.5h; | 100% |
| biscobalthexacarbonyl-2-methyl-but-3-yn-2-ol In 1,2-dichloro-ethane |
-
-
617-86-7
triethylsilane

-
-
109660-11-9
4,4-dimethyl-2-(naphthalen-2-yl)-4,5-dihydrooxazole

| Conditions | Yield |
|---|---|
| With norborn-2-ene; dodecacarbonyl-triangulo-triruthenium In toluene for 20h; Heating; | 100% |
-
-
617-86-7
triethylsilane

| Conditions | Yield |
|---|---|
| With norborn-2-ene; dodecacarbonyl-triangulo-triruthenium In toluene for 20h; Heating; | 100% |
| With norborn-2-ene; dodecacarbonyl-triangulo-triruthenium In toluene for 36h; Inert atmosphere; Schlenk technique; Reflux; | 59.6 mg |

| Conditions | Yield |
|---|---|
| AuCl(xanthphos) In acetone at 50℃; for 8h; | 100% |
| With copper (I) tert-butoxide; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 25℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| copper dichloride at 200℃; for 45h; | 100% |
| copper diacetate at 200℃; for 45h; | 100% |
| copper(II) sulfate at 200℃; for 45h; | 100% |
Triethylsilane Specification
The Triethylsilane is an organic compound with the formula C6H16Si. The IUPAC name of this chemical is triethylsilicon. With the CAS registry number 617-86-7, it is also named as Silane, triethyl-. The product's categories are Other Reagents; Reduction; Si (Classes of Silicon Compounds); Si-H Compounds; Silicon Compounds (for Synthesis); Synthetic Organic Chemistry; Alkyl Silanes; Hydrogensilanes Hydrogensiloxanes; Reducing Agents; Chemistry; Silanes; Synthetic Reagents. Besides, it is clear liquid, which should be stored in a dark closed and dry place. It is often used in organic synthesis, specifically for the hydrosilation of olefins to give alkyl silanes. It can also be used as a reducing agent since it has an active hydride.
Physical properties about Triethylsilane are: (1)ACD/LogP: 3.65; (2)ACD/LogD (pH 5.5): 3.65; (3)ACD/LogD (pH 7.4): 3.65; (4)ACD/BCF (pH 5.5): 349.789; (5)ACD/BCF (pH 7.4): 349.789; (6)ACD/KOC (pH 5.5): 2303.888; (7)ACD/KOC (pH 7.4): 2303.888; (8)#Freely Rotating Bonds: 3; (9)Enthalpy of Vaporization: 33.2 kJ/mol; (10)Boiling Point: 107.499 °C at 760 mmHg; (11)Vapour Pressure: 31.55 mmHg at 25°C.
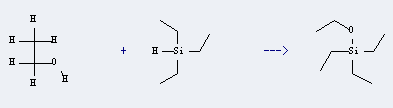
Uses of Triethylsilane: it can be used to produce ethoxy-triethyl-silane. It will need reagent NaOEt.
When you are using this chemical, please be cautious about it as the following:
It is highly flammable. Please keep away from sources of ignition - No smoking. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable gloves and eye/face protection, do not breathe dust and take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)SMILES: CC[SiH](CC)CC
(2)InChI: InChI=1/C6H16Si/c1-4-7(5-2)6-3/h7H,4-6H2,1-3H3
(3)InChIKey: AQRLNPVMDITEJU-UHFFFAOYAL
(4)Std. InChI: InChI=1S/C6H16Si/c1-4-7(5-2)6-3/h7H,4-6H2,1-3H3
(5)Std. InChIKey: AQRLNPVMDITEJU-UHFFFAOYSA-N
Related Products
- Triethylsilane
- 617880-45-2
- 61788-32-7
- 61788-33-8
- 61788-40-7
- 61788-44-1
- 61788-45-2
- 61788-46-3
- 61788-47-4
- 61788-48-5
- 61788-49-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View