-
Name
Urethane
- EINECS 200-123-1
- CAS No. 51-79-6
- Article Data185
- CAS DataBase
- Density 1.045 g/cm3
- Solubility slightly soluble
- Melting Point 48-50 °C(lit.)
- Formula C3H7NO2
- Boiling Point 184 °C at 760 mmHg
- Molecular Weight 89.0941
- Flash Point 97.2 °C
- Transport Information
- Appearance COA
- Safety 53-45-99
- Risk Codes 45
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Ethylaminoformate;Ethyl carbamate;Ethylurethane;Leucethane;O-Ethylurethane;Pracarbamine;
- PSA 52.32000
- LogP 0.80190
Synthetic route
-
-
110-82-7
cyclohexane

-
-
66121-61-7
N,O-bistrimethylsilyl-N-(ethoxycarbonyl)hydroxylamine

-
A
-
107-46-0
Hexamethyldisiloxane

-
B
-
1541-19-1
ethyl N-cyclohexylcarbamate

-
C
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| at 100℃; for 25h; | A 100% B 80% C 8% |
| at 45℃; for 58h; Irradiation; | A 75% B 45% C 55% |


| Conditions | Yield |
|---|---|
| With alumina supported chromium oxide-nickel oxide-bismuth oxide trimetal oxide catalyst at 90℃; for 12h; Sealed tube; | 99.1% |
| at 179.84℃; for 7h; Autoclave; | 90% |
-
-
75934-53-1
ethyl-N-(carboethoxy)-N-nitrocarbamate

-
A
-
62258-40-6
N-nitrouretahne ammonium salt

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With ammonia In acetonitrile for 0.0833333h; | A 99% B 0.15 g |

-
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| sodium hydrogencarbonate | 96% |
-
-
24482-75-5
Dichloronitroacetic acid ethyl ester

-
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With ammonium hydroxide for 48h; Ambient temperature; | 91% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at -2 - 2℃; for 6h; | 90% |
| With perchloric acid on silica gel at 20℃; for 0.75h; | 73% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; Petroleum ether; benzene | 90% |

-
A
-
36600-01-8
N-methyl-N-octylformamide

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With acetic acid In 1,4-dioxane at 20℃; for 1h; | A 89% B n/a |

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate In acetonitrile preparative electrolyse, - 2.3 V; | A 87% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With ethanol; carbon monoxide In ethanol to degassed ethanol educt added with stirring while CO bubled through for 15 h; product filtered off, washed with ethanol and hexane, dried in vac.; elem. anal.; | A 85% B n/a |

-
A
-
76058-02-1
N-methyl-N-1-dodecylformamide

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With acetic acid In 1,4-dioxane at 20℃; for 1h; | A 83% B n/a |

| Conditions | Yield |
|---|---|
| With di(n-butyl)tin oxide at 140℃; for 12h; Inert atmosphere; | A 80% B 80% |
| With di(n-butyl)tin oxide at 140℃; under 2585.81 Torr; for 12h; Inert atmosphere; Autoclave; | A 30 %Chromat. B 60 %Chromat. |


| Conditions | Yield |
|---|---|
| With para-dodecylbenzenesulfonic acid In neat (no solvent) at 60℃; for 0.5h; Green chemistry; | 79% |
| With hydrogenchloride Darst, dann neutralisiert man mit Bariumcarbonat; | |
| With DBSA at 60℃; for 1h; | |
| With DBSA at 60℃; for 1h; | |
| With DBSA at 60℃; for 1h; |
-
-
90454-50-5
N-ethoxycarbonyl-(2,3,4,5-tetrachloro-1-thiophenio)amide

-
A
-
6012-97-1
2,3,4,5-tetrachlorothiophene

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In tetrahydrofuran for 4h; | A 61% B 68% |
| With hydrogen; nickel Product distribution; | A n/a B 68% |

| Conditions | Yield |
|---|---|
| With MgZn1.7Al hydrotalcite calcined at 450°C at 200℃; under 15001.5 Torr; for 4h; Catalytic behavior; Reagent/catalyst; Autoclave; | A 18% B 68% |
| With calcined Y(NO3)3x6H2O; calcined Y(NO3)3x6H2O at 180℃; under 11096.7 Torr; for 4h; Inert atmosphere; Autoclave; | A 11.2% B 62.4% |
| With Mg2Zr0.53Al0.47 mixed metal oxides at 200℃; for 5h; Reagent/catalyst; Temperature; | A 45.3% B 37.6% |

-
-
109-02-4
4-methyl-morpholine

-
-
817-87-8
ethyl azidocarbonate

-
A
-
58050-49-0
4-(ethoxycarbonylaminomethyl)morpholine

-
B
-
75256-50-7
4-methyl-3-(ethoxycarbonylamino)morpholine

-
C
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| at 115℃; for 3h; Further byproducts given; | A 13.4% B 2.5% C 61.1% |

-
-
64-17-5
ethanol

-
-
2911-21-9
2,4-dioxo-6-methyl-3,4-dihydro-2H-1,3-oxazine

-
A
-
141-97-9
ethyl acetoacetate

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With triethylamine for 24h; Heating; | A 60% B 12% |
| With triethylamine Heating; Yield given; |

-
-
66121-61-7
N,O-bistrimethylsilyl-N-(ethoxycarbonyl)hydroxylamine

-
-
71-43-2
benzene

-
A
-
2955-79-5
N-ethoxycarbonylazepine

-
B
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| at 90℃; for 75h; | A 57% B n/a |

-
-
625-57-0
O-ethyl thiocarbamate

-
A
-
4115-25-7
3,5-diethoxy-1,2,4-thiadiazole

-
B
-
75-00-3
chloroethane

-
C
-
51-79-6
urethane

-
D
-
178318-21-3
3-ethoxy-1,2,4-dithiazole-5-one

| Conditions | Yield |
|---|---|
| With chloro(chlorosulfanyl)methanone; triethylamine In chloroform at 5 - 10℃; for 0.25h; | A 54% B 17% C 3% D 7% |

-
-
817-87-8
ethyl azidocarbonate

-
-
106-42-3
para-xylene

-
A
-
30559-02-5
2,5-dimethyl-azepine-1-carboxylic acid ethyl ester

-
B
-
76917-05-0
ethyl 2,5-dimethylphenylcarbamate

-
C
-
76917-04-9
3,6-Dimethyl-azepine-1-carboxylic acid ethyl ester

-
D
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| Heating; | A 53% B 7% C 34% D 2% |
| cobalt(II) 5,10,15,20-tetraphenylporphyrin Product distribution; Heating; others catalysts; |

-
-
75633-16-8
1-ethoxycarbonyl-5-methyl-1H-1,3-diazepine

-
A
-
37772-60-4
5-hydroxy-3-methyl-1,5-dihydropyrrol-2-one

-
B
-
18804-91-6
ethyl N-carbonylcarbamate

-
C
-
91024-61-2
1-formyl-5-hydroxy-3-methyl-3-pyrrolin-2-one

-
D
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With 5,10,15,20-tetrakisphenylporphyrin; oxygen In tetrachloromethane for 2h; Ambient temperature; Irradiation; | A 53% B 32% C 19% D 18% |
| With 5,10,15,20-tetrakisphenylporphyrin; water; oxygen 1.) carbon tetrachloride, irradiation, 30 min; 2.) tetrahydrofuran, r.t., 6 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
20160-60-5
2-(trimethylsilyl)-ethoxycarbonyl chloride

-
-
5468-37-1
2-aminoacetophenone hydrochloride

-
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 53% |
| With triethylamine In tetrahydrofuran | 53% |
-
-
57492-79-2
N-(2,4-dimethyl-6,7-methylenedioxy-3-quinazolinio)-ethoxyformamidate

-
A
-
66117-80-4
(6-methyl-[1,3]dioxolo[4,5-g]quinazolin-8-ylmethyl)-carbamic acid ethyl ester

-
B
-
66117-79-1
1,2-bis(2-methyl-6,7-methylenedioxy-4-quinazolinyl)ethane

-
C
-
51-79-6
urethane

-
E
-
57492-84-9
2,4-dimethyl-6,7-methylenedioxyquinazoline

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 80h; Mechanism; Heating; other solvent and temperature, other substrate; | A 5% B 1.4% C n/a D 15% E 51% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In benzene at 70 - 75℃; for 3.5h; | 100% |
| With barium dihydroxide In water for 40h; Ambient temperature; | 38% |
| With barium dihydroxide |
-
-
684-16-2
Hexafluoroacetone

-
-
51-79-6
urethane

-
-
19396-71-5
(2,2,2-Trifluoro-1-hydroxy-1-trifluoromethyl-ethyl)-carbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| for 72h; Ambient temperature; | 100% |
| With toluene-4-sulfonic acid In dichloromethane |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; tributylphosphine In dichloromethane at 20℃; for 15h; aza-Michael reaction; | 100% |
| With boron trifluoride diethyl etherate; tetrabutylammomium bromide In dichloromethane at 20℃; for 24h; aza-Michael reaction; | 95% |
| With chloro-trimethyl-silane; iron(III) chloride In dichloromethane at 20℃; for 10h; aza-Michael reaction; | 93% |

-
-
51-79-6
urethane

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In toluene at 110℃; for 2h; | 100% |
-
-
1246541-59-2
bis[4-(docosyloxy)phenyl]methyl alcohol

-
-
51-79-6
urethane

-
-
1394992-05-2
ethyl [bis-(4-docosoxyphenyl)methyl]carbamate

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In toluene at 110℃; for 2.5h; | 100% |
| With methanesulfonic acid In toluene at 110℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 3h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; urethane With sulfuric acid; water at 20℃; for 0.166667h; Stage #2: colchicine at 20℃; for 4h; | 99% |

-
-
4663-33-6, 62668-02-4, 62839-70-7, 116127-91-4, 132014-29-0, 136981-81-2, 148616-45-9
1,3-diphenyl-3-hydroxypropene

-
-
51-79-6
urethane

-
-
1374561-60-0
(E)-ethyl (1,3-diphenylallyl)carbamate

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride In dichloromethane at 20℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); sodium t-butanolate In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; bis(η3-allyl-μ-chloropalladium(II)); (2S,3S,5R,6R)-2,3-bis(2-(diphenylphosphino)phenyl)-1,4-dimethyldecahydroquinoxaline In N,N-dimethyl acetamide at 20℃; for 12h; enantioselective reaction; | A 99% B n/a |

-
-
1745-46-6
6,11-dihydrodibenzo[b,e]thiepin-11-ol

-
-
51-79-6
urethane

-
-
74797-18-5
ethyl N-(6,11-dihydrodibenzothiepin-11-yl)carbamate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetic acid 1) 37 deg C, 6h; 2) room temp., overnight; | 98% |
-
-
53662-58-1
1,1-dimethoxy-pentan-2-ol

-
-
51-79-6
urethane

-
-
104681-97-2
(2-Oxo-pentyl)-carbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In benzene for 1h; Heating; | 98% |
-
-
124-09-4
1,6-Hexanediamine

-
-
64-17-5
ethanol

-
-
51-79-6
urethane

-
-
3066-65-7
1,6-bis-(ethoxycarbonylamino)-hexane

| Conditions | Yield |
|---|---|
| With 5.1 wt% Ni/Fe3O4 at 190℃; under 11251.1 - 16501.7 Torr; for 5h; Autoclave; Inert atmosphere; | 98% |


| Conditions | Yield |
|---|---|
| With 5.1 wt% Ni/Fe3O4 at 190℃; under 11251.1 - 16501.7 Torr; for 5h; Autoclave; Inert atmosphere; | 98% |


| Conditions | Yield |
|---|---|
| With 5.1 wt% Ni/Fe3O4 at 190℃; under 11251.1 - 16501.7 Torr; for 5h; Autoclave; Inert atmosphere; | 98% |


| Conditions | Yield |
|---|---|
| With 1-methyl-imidazolium p-toluenesulfonic acid at 20℃; for 16h; Michael addition; neat (no solvent); | 98% |
-
-
7745-93-9
2-bromo-4-nitrotoluene

-
-
51-79-6
urethane

-
-
16648-52-5
ethyl (2-methyl-5-nitrophenyl)carbamate

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); triisopropylsilanol; potassium hydroxide In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 98% |
-
-
90-90-4
(4-bromophenyl)(phenyl)methanone

-
-
51-79-6
urethane

-
-
289471-18-7
ethyl (4-benzoylphenyl)carbamate

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); sodium t-butanolate In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 98% |
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); TPGS-750-M; sodium t-butanolate In water at 50℃; for 24h; Reagent/catalyst; Inert atmosphere; Sealed tube; | 96% |
-
-
292638-84-7
styrene

-
-
39652-06-7
benzenetellurinyl acetate

-
-
51-79-6
urethane

-
-
112476-38-7
ethyl <1-phenyl-2-(phenyltelluro)ethyl>carbamate

| Conditions | Yield |
|---|---|
| boron trifluoride diethyl etherate In chloroform for 20h; Product distribution; Heating; further educts and catalysts; | 97% |
| boron trifluoride diethyl etherate In chloroform for 20h; Heating; | 97% |
| With boron trifluoride diethyl etherate; hydrazine hydrate 1.) CHCl3, reflux, 20 h; 2.) EtOH, 60 deg C, 15 min; Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With air; 4-nitro-benzoic acid; [(S,S)-N,N’-bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]cobalt(II) In various solvent(s) at 20℃; for 24h; | A n/a B 97% |

-
-
51-79-6
urethane

-
-
177896-09-2
tert-butyl benzylidenecarbamate

| Conditions | Yield |
|---|---|
| With bis(trifluoromethanesulfonyl)amide In diethyl ether at 20℃; for 0.333333h; | 97% |
-
-
99-61-6
3-nitro-benzaldehyde

-
-
51-79-6
urethane

-
-
135-19-3
β-naphthol

-
-
1372764-64-1
ethyl ((3-nitrophenyl)(2-hydroxynaphthalen-1-yl)methyl) carbamate

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate In neat (no solvent) at 70℃; for 0.5h; | 97% |
| With tin (IV) chloride pentahydrate In neat (no solvent) at 60℃; for 0.1h; | 95% |
| With 1-methyl-2-oxopyrrolidinium hydrogen sulfate at 125℃; for 0.15h; | 93% |
| With Tween 20 In water at 75 - 80℃; for 0.5h; | 90% |

-
-
104-95-0
(4-bromophenyl)thioanisole

-
-
51-79-6
urethane

-
-
501679-13-6
ethyl (4-methylthiophenyl)carbamate

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); triisopropylsilanol; potassium hydroxide In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 97% |
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
51-79-6
urethane

-
-
20131-85-5
N-(4-formylphenyl)carbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran at 80℃; | 96.3% |

| Conditions | Yield |
|---|---|
| In toluene | 96% |

Urethane Consensus Reports
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1974,p. 111.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Community Right-To-Know List. Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Urethane Standards and Recommendations
DFG MAK: Animal Carcinogen, Suspected Human Carcinogen
Urethane Specification
The Urethane with CAS registry number of 51-79-6 is also called Carbamic acid, ethylester. Its EINECS registry number is 200-123-1. The IUPAC name is ethyl carbamate. In addition, the molecular formula is C3H7NO2 and the molecular weight is 89.09. It is a kind of white crystalline powder. And it is incompatible with strong acids, strong bases and strong oxidizing agents.
Physical properties about this chemical are: (1)ACD/LogP: -0.15; (2)ACD/LogD (pH 5.5): -0.15; (3)ACD/LogD (pH 7.4): -0.15; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 19.74; (7)ACD/KOC (pH 7.4): 19.74; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 2; (11)Polar Surface Area: 29.54 Å2; (12)Index of Refraction: 1.413; (13)Molar Refractivity: 21.25 cm3; (14)Molar Volume: 85.2 cm3; (15)Polarizability: 8.42 ×10-24cm3; (16)Surface Tension: 33.6 dyne/cm; (17)Density: 1.045 g/cm3; (18)Flash Point: 97.2 °C; (19)Enthalpy of Vaporization: 42.03 kJ/mol; (20)Boiling Point: 184 °C at 760 mmHg; (21)Vapour Pressure: 0.748 mmHg at 25°C.
Preparation of Urethane: it can be prepared by urea nitrate, ethanol and sodium nitrite in the presence of sulfuric acid. This reaction is a kind of esterification reaction. Then go through the operation of distillation, crystallization and drying to get the products. In addition, it can be prepared by ethanol and cyanic acid;sodium cyanate. This reaction will need reagent HCl and solvent CH2Cl2. The reaction time is 6 hours at reaction temperature of -2 - 2 °C. The yield is about 90%.
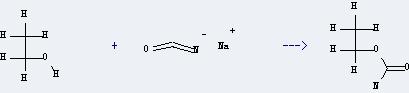
Uses of Urethane: this chemical can be used as intermediates for medicine, pesticides and perfume. And it is used for biochemical research and the production of sleeping pills and tranquilizers. Moreover, it can be used as fungicides, cosolvent of injection and colorants in dyeing industry. In addition, it can be used to get isocyanatophosphoric acid-dichloride. This reaction will need reagent PCl5 and solvent CH2Cl2. The reaction time is 4 hours by heating. The yield is about 65%.
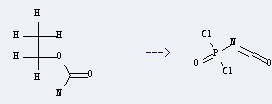
When you are using this chemical, please be cautious about it as the following:
This chemical may cause cancer. Avoid exposure-obtain special instructions before use. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.). In addition, you should keep container in a well-ventilated place.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)N
(2)InChI: InChI=1/C3H7NO2/c1-2-6-3(4)5/h2H2,1H3,(H2,4,5)
(3)InChIKey: JOYRKODLDBILNP-UHFFFAOYAY
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| chicken | LDLo | oral | 2188mg/kg (2188mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| guinea pig | LDLo | intraarterial | 800mg/kg (800mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 131, Pg. 171, 1928. | |
| guinea pig | LDLo | intravenous | 800mg/kg (800mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 131, Pg. 171, 1928. | |
| mouse | LD50 | intraperitoneal | 1539mg/kg (1539mg/kg) | Progress in Mutation Research. Vol. 1, Pg. 682, 1981. | |
| mouse | LD50 | intravenous | 500mg/kg (500mg/kg) | Acta Pharmaceutica Jugoslavica. Vol. 5, Pg. 43, 1955. | |
| mouse | LD50 | oral | 2500mg/kg (2500mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 9, Pg. 595, 1959. | |
| mouse | LD50 | subcutaneous | 1750mg/kg (1750mg/kg) | Gann. Japanese Journal of Cancer Research. Vol. 63, Pg. 731, 1972. | |
| mouse | LD50 | unreported | 2gm/kg (2000mg/kg) | British Journal of Cancer. Vol. 6, Pg. 160, 1952. | |
| mouse | LDLo | parenteral | 1gm/kg (1000mg/kg) | GASTROINTESTINAL: PERITONITIS | Progress Report for Contract No. PH-43-63-1132, Submitted to the National Cancer Institute by Scientific Associates, Inc. Vol. PH-43-62-483, |
| pigeon | LDLo | oral | 800mg/kg (800mg/kg) | "Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. | |
| rabbit | LDLo | intravenous | 2gm/kg (2000mg/kg) | "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 272, 1973. | |
| rat | LD50 | intramuscular | 1400mg/kg (1400mg/kg) | Zeitschrift fuer Krebsforschung und Klinische Onkologie. Vol. 84, Pg. 227, 1975. | |
| rat | LD50 | intraperitoneal | 1500mg/kg (1500mg/kg) | Cancer Research. Vol. 26, Pg. 1448, 1966. | |
| rat | LD50 | oral | 1809mg/kg (1809mg/kg) | Cancer Letters Vol. 57, Pg. 37, 1991. | |
| rat | LDLo | subcutaneous | 1800mg/kg (1800mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 182, Pg. 348, 1936. |
Related Products
- Urethane
- Urethane dimethacrylate
- 51798-45-9
- 51801-29-7
- 51801-69-5
- 51802-42-7
- 51802-78-9
- 518033-33-5
- 51803-38-4
- 51803-78-2
- 518044-40-1
- 518046-08-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View