-
Name
Zidovudine
- EINECS 623-849-4
- CAS No. 30516-87-1
- Article Data72
- CAS DataBase
- Density 1.3382 (rough estimate)
- Solubility 1-5 g/100 mL at 17 °C in water
- Melting Point 106-112 °C
- Formula C10H13N5O4
- Boiling Point 410.43°C (rough estimate)
- Molecular Weight 267.244
- Flash Point 9℃
- Transport Information
- Appearance Off -white crystalline powder
- Safety 36/37/39-45-36-26
- Risk Codes 40-36/37/38-20/21/22
-
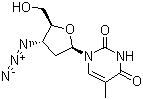
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 3-Azido-3-deoxythymidine;3'-Azido-3'-deoxythymidine;3'-Azidothymidine;3'-Deoxy-3'-azidothymidine;AZT;AZT (pharmaceutical);Azidothymidine;Azitidin;BW-A 509U;Compound S;NSC602670;Retrovir;Retrovir IV;Retrovis;Timazid;Viro-Z;ZDV;ZVD;Zido-H;
- PSA 134.07000
- LogP -0.74354
Synthetic route
-
-
106060-78-0
3'-azido-5'-O-benzoyl-3'-deoxythymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 24h; Ambient temperature; | 95% |
| With ammonia In methanol at 20℃; | 81% |
| With sodium methylate In methanol | 71% |
| With ammonia In methanol |
-
-
29706-84-1
1-(3-azido-2,3-deoxy-5-O-trityl-β-D-erythro-pentofuranosyl)thymine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With silica gel; trifluoroacetic acid In methanol; chloroform | 95% |
| With hydrogenchloride In methanol; water at 20℃; for 3h; | 95% |
| With hydrogenchloride In acetic acid at 20℃; for 2h; | 49% |
| With sodium periodate In acetone at 50℃; for 20h; |
-
-
134077-32-0
3'-Azido-5'-O-(4-methoxybenzoyl)-3'-deoxythymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 12h; Ambient temperature; | 94% |
| With sodium methylate In methanol |
-
-
162404-30-0
AZT guanidine salt

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 75℃; Large scale reaction; | 88.5% |
-
-
126441-74-5
3’-azido-5’-O-(4,4’-dimethoxytrityl)-3’-deoxythymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In methanol; tetrachloromethane at 25 - 40℃; for 12h; ultrasonic; | 87% |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20℃; for 1h; | 86% |
-
-
52450-18-7
3'-amino-3'-deoxythymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With fluorosulfonyl azide; potassium hydrogencarbonate In tert-butyl methyl ether; water; N,N-dimethyl-formamide at 20℃; for 4h; | 83% |
-
-
15981-92-7
2,3'-anhydrothymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide for 3h; Heating; | 71% |
-
-
15981-92-7
2,3'-anhydrothymidine

-
A
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With lithium azide In N,N-dimethyl-formamide at 110℃; for 24h; | A 56% B n/a |
| With lithium azide In N,N-dimethyl-formamide at 110℃; for 24h; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 0.5h; Ambient temperature; | A 31% B 45% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Yield given. Yields of byproduct given; | |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Yield given; | |
| With tetrabutyl ammonium fluoride In tetrahydrofuran |
-
-
7288-28-0
2,4-bis(trimethylsiloxy)-5-methylpyrimidine

-
-
371164-35-1
4,5-O-cyclohexylidene-2,3-dideoxy-3-azido-1,1-dimethoxy-D-glycero-pentanose

-
A
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
B
-
73971-82-1
1-(3'-azido-2',3'-dideoxy-β-D-threo-pentofuranosyl)thymine

-
D
-
66323-40-8
alpha-3'-azido-2',3'-dideoxythymidine

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In acetonitrile at -30 - 20℃; Further byproducts given; | A 38% B 13% C 12% D 25% |


| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 20h; Ambient temperature; | A 33% B 29% |

-
A
-
99004-90-7
3'-amino-3'-deoxythymidine hydrochloride

-
B
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With α,α'-azodiizobutyramidine-dihydrochloride In water-d2; d(4)-methanol at 50℃; for 24h; Inert atmosphere; | A Ca. 5 mg B 25% |
-
-
25442-42-6
5'-O-trityl-2,3'-anhydrothymidine

-
-
29706-84-1
1-(3-azido-2,3-deoxy-5-O-trityl-β-D-erythro-pentofuranosyl)thymine

-
A
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
B
-
65-71-4
thymin

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 108℃; for 24h; | A 31 %Chromat. B 16 %Chromat. C 8.6% |

-
-
25442-42-6
5'-O-trityl-2,3'-anhydrothymidine

-
A
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With lithium azide; acetic acid 1.) DMF, 150 deg C, 3 h, 2.) 55 deg C, 4 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
66323-42-0
1-(5′-O-acetyl-3′-azido-2′,3′-dideoxy-β-D-ribofuranosyl)thymine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |
-
-
130683-74-8
3‘-azido-2’,3’-dideoxy-5’-O-(tetradecanoyl)thymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| In ethanol at 40℃; Rate constant; chemical and enzymatic hydrolysis; var. buffers pH (2.0-9.0); var. temperature; |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol Yield given; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Yield given. Yields of byproduct given; |
-
-
123533-06-2
3'-azido-3'-deoxy-5'-O-thexyldimethylsilylthymidine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With Dowex 50(H+) In methanol | |
| With Dowex 50 x 2 (H+) In methanol Ambient temperature; |
-
-
136011-36-4
1-(3-azido-2,3-dideoxy-β-D-ribo-hexofuranosyl)thymine

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With Dowex 1(BH4-); Dowex 1(IO4-) 1) 80percent aq. MeOH, r.t., 1.5 h, 2) r.t., 1.5 h; Yield given. Multistep reaction; |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
147907-41-3
phenyl methoxyleucinyl phosphorochloridate

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In tetrahydrofuran for 5h; Ambient temperature; | 100% |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With pentaisopropylguanidine In chloroform-d1 addn. of Zn complex to soln. of pentaisopropylguanidine and azidodeoxythymidine; (1)H-NMR-monitoring; | 100% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrachloromethane; 1,2-dichloro-ethane; acetonitrile at 20℃; for 24h; | 100% |
-
-
7693-45-0
4-Chlorophenyl chloroformate

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1355331-53-1
C17H16ClN5O6

| Conditions | Yield |
|---|---|
| With pyridine | 100% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
133101-33-4
1,2,3,4-tetra-O-acetyl-6-D-glucose phosphate pyridinium form

| Conditions | Yield |
|---|---|
| In pyridine | 99% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
147907-40-2
phenyl (methoxyglycinyl)phosphorochloridate

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In tetrahydrofuran for 12h; Ambient temperature; | 99% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
108441-46-9
3'-azido-3'-deoxy-3-methylthymidine

| Conditions | Yield |
|---|---|
| In diethyl ether; ethanol at 0℃; for 0.5h; | 99% |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; sodium L-ascorbate In water; tert-butyl alcohol at 20℃; for 12h; Inert atmosphere; Schlenk technique; | 99% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1609-47-8
diethyl dicarbonate

| Conditions | Yield |
|---|---|
| With dmap In pyridine for 1h; Ambient temperature; | 98% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
147907-39-9
(4-fluorophenoxy)(((1S)-(1-methoxycarbonylethyl))amino)phosphinylchloride

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In tetrahydrofuran for 12h; Ambient temperature; | 97% |
| With 1-methyl-1H-imidazole In tetrahydrofuran |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
52450-18-7
3'-amino-3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol | 97% |
| With hydrogen; palladium on activated charcoal In methanol | 92% |
| With hydrogen; palladium on activated charcoal at 20℃; for 6h; Reduction; | 87% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
64638-13-7
3'-Azido-3',5'-dideoxythymidine-5'-O-tosylthymidine

| Conditions | Yield |
|---|---|
| With pyridine for 96h; Ambient temperature; | 97% |
| With pyridine | 96% |
| With pyridine Ambient temperature; | 74% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1048636-94-7
2',5'-dideoxy-5'-ethynyl-3'-epi-thymidine

| Conditions | Yield |
|---|---|
| With copper bromide dimethyl sulfide complex In tetrahydrofuran; water; tert-butyl alcohol at 80℃; for 1h; Huisgen reaction; Microwave irradiation; | 97% |
| With dimethylsulfide; triethylamine In tetrahydrofuran; water; tert-butyl alcohol at 80℃; for 1h; Microwave irradiation; | 25.1 mg |
-
-
1033098-90-6
2-propyn-1-yl 2,3,4-tri-O-acetyl-β-D-xylopyranose

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1108170-91-7
C24H31N5O12

| Conditions | Yield |
|---|---|
| With copper(II) sulfate; sodium L-ascorbate In tetrahydrofuran; water at 20℃; for 24h; Huisgen cycloaddition; | 96.9% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
147907-38-8
(4‐methoxy)phenyl (methoxy‐L‐alaninyl)phosphorochloridate

-
-
149560-32-7
3'-azidothymidine 5'-[p-methoxyphenyl methoxyalaninyl phosphate]

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In tetrahydrofuran for 16h; Ambient temperature; | 96% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
108-24-7
acetic anhydride

-
-
66323-42-0
1-(5′-O-acetyl-3′-azido-2′,3′-dideoxy-β-D-ribofuranosyl)thymine

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 96% |
| With pyridine at 20℃; for 8h; | 94% |
| With pyridine Ambient temperature; |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
67373-56-2
chlorodimethyl(1,1,2-trimethylpropyl)silane

-
-
123533-06-2
3'-azido-3'-deoxy-5'-O-thexyldimethylsilylthymidine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 2h; Ambient temperature; | 96% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
120624-97-7
1-{(2R,4S,5S)-4-azido-5-{{[(1,1-dimethylethyl)dimethylsilyl]oxy}methyl}tetrahydrofuran-2-yl}-5-methylpyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide | 96% |
| With 1H-imidazole In acetonitrile at 20℃; | 92% |
| With 1H-imidazole at 20℃; for 12h; Inert atmosphere; | 91% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
34272-02-1
1-O-propargyl 2,3,4,6-tetra-O-acetyl-β-D-glucose

-
-
1108170-87-1
C27H35N5O14

| Conditions | Yield |
|---|---|
| With copper(II) sulfate; sodium L-ascorbate In tetrahydrofuran; water at 20℃; for 24h; Huisgen cycloaddition; | 96% |
-
-
766-98-3
1-ethynyl-4-fluorobenzene

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
127479-75-8
3′-deoxy-3′-[4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl]-thymidine

| Conditions | Yield |
|---|---|
| With copper; copper(II) sulfate In water; tert-butyl alcohol at 125℃; for 0.333333h; Huisgen 1,3-dipolar cycloaddition; Microwave irradiation; regioselective reaction; | 96% |
| With copper(ll) sulfate pentahydrate; L-ascorbic acid sodium salt In tetrahydrofuran; water at 20℃; for 12h; |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
107-19-7
propargyl alcohol

-
-
127479-69-0
1-((2R,4S,5S)-5-(hydroxymethyl)-4-(4-(hydroxymethyl)-1H-1,2,3-triazol-1-yl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; sodium L-ascorbate In tetrahydrofuran; water at 20℃; for 12h; Huisgen Cycloaddition; Inert atmosphere; | 96% |
| With copper; copper(II) sulfate In water; tert-butyl alcohol at 125℃; for 0.166667h; Huisgen 1,3-dipolar cycloaddition; Microwave irradiation; regioselective reaction; | 80% |
| With copper(ll) sulfate pentahydrate; sodium L-ascorbate In tetrahydrofuran; water at 20℃; for 12h; Huisgen Cycloaddition; |
-
-
17061-86-8
1-methoxy-4-(2-propynyloxy)benzene

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1310031-49-2
C20H23N5O6

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-4-(2-propynyloxy)benzene; 3'-azido-2',3'-deoxythymidine In water; tert-butyl alcohol at 20℃; for 0.166667h; Stage #2: With copper(II) sulfate; sodium L-ascorbate In water; tert-butyl alcohol at 20℃; | 96% |
-
-
20009-28-3
naphth-2-yloxymethylacetylene

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
1310031-51-6
1-((2R,4S,5S)-5-(hydroxymethyl)-4-(4-((naphthalen-2-yloxy)-methyl)-1H-1,2,3-triazol-1-yl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| Stage #1: naphth-2-yloxymethylacetylene; 3'-azido-2',3'-deoxythymidine In water; tert-butyl alcohol at 20℃; for 0.166667h; Stage #2: With copper(II) sulfate; sodium L-ascorbate In water; tert-butyl alcohol at 20℃; | 96% |
| With copper(ll) sulfate pentahydrate; sodium L-ascorbate In tetrahydrofuran; water at 20℃; for 12h; Huisgen Cycloaddition; Inert atmosphere; | 84% |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With copper(II) acetate monohydrate; sodium L-ascorbate In isopropyl alcohol at 45℃; for 0.166667h; Wavelength; UV-irradiation; | 96% |

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; sodium L-ascorbate In dimethyl sulfoxide at 20℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| With copper(I) thiophene-2-carboxylate In toluene at 50℃; | 96% |
-
-
108-30-5
succinic acid anhydride

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
106060-83-7
4-((3-azido-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methoxy)-4-oxobutanoic acid

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 20℃; for 18h; | 95% |
| With pyridine; dmap at 20℃; for 4h; | 94% |
| With dmap In N,N-dimethyl-formamide at 20℃; for 14h; | 85% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

-
-
88-10-8
N,N-diethylcarbamyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 36h; Ambient temperature; | 95% |
-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile Condensation; | 95% |
Zidovudine History
Jerome Horwitz of Barbara Ann Karmanos Cancer Institute and Wayne State University School of Medicine first synthesized Zidovudine in 1964, under a US National Institutes of Health (NIH) grant. Zidovudine was originally intended as an anticancer drug, but was shelved after it proved insufficiently toxic to cancer tumours in mice.
Zidovudine Specification
The IUPAC name of Zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione. With the CAS registry number 30516-87-1, it is also named as 1-(3-Azido-2,3-dideoxy-beta-D-ribofuranosyl)-5-methylpyrimidine-2,4-(1H,3H)-dione. It is off -white crystalline powder which is a dideoxynucleoside compound in which the 3'-hydroxy group on the sugar moiety has been replaced by an azido group. When burns, it will produce toxic nitrogen oxide fumes. Additionally, this chemical should be sealed in the container and stored at -20 °C.
The other characteristics of this product can be summarized as: (1)ACD/LogP: -0.53; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.53; (4)ACD/LogD (pH 7.4): -0.53; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 12.29; (8)ACD/KOC (pH 7.4): 12.12; (9)#H bond acceptors: 9; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 71.44 Å2; (13)Rotatable Bond Count: 3; (14)Tautomer: Count 3; (15)Exact Mass: 267.096754; (16)MonoIsotopic Mass: 267.096754; (17)Topological Polar Surface Area: 93.2; (18)Heavy Atom Count: 19; (19)Complexity: 484; (20)Defined Atom StereoCenter Count: 3.
Preparation of Zidovudine: It can be obtained by thymidine, triphenylphosphine, p-methoxybenzo and Diethyl azodicarboxylate.
.gif)
.gif)
Uses of Zidovudine: It is a type of antiretroviral drug used for the treatment of HIV/AIDS. Its use was a major breakthrough in AIDS therapy in the 1990s that significantly altered the course of the illness and helped destroy the notion that HIV/AIDS was a death sentence. This drug is a potent inhibitor of HIV replication, acting as a chain-terminator of viral DNA during reverse transcription. It improves immunologic function, partially reverses the HIV-induced neurological dysfunction, and improves certain other clinical abnormalities associated with AIDS. Chronic, high-dose therapy with Zidovudine is associated with significant side effects, including anemia, neutropenia, hepatotoxicity, cardiomyopathy, and myopathy.
When you are using this chemical, please be cautious about it as the following:
It is not only harmful by inhalation, in contact with skin and if swallowed, but also irritating to eyes, respiratory system and skin. It also has limited evidence of a carcinogenic effect. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
1. Smiles:n1([C@H]2C[C@H](N=[N+]=[N-])[C@H](O2)CO)c([nH]c(=O)c(c1)C)=O
2. InChI:InChI=1/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | LDLo | oral | 86mg/kg/1W-I (86mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: HEADACHE | Lancet. Vol. 2, Pg. 1392, 1986. |
| man | TDLo | oral | 434mg/kg/38D- (434mg/kg) | BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE BLOOD: APLASTIC ANEMIA | Annals of Internal Medicine. Vol. 107, Pg. 502, 1987. |
| man | TDLo | unreported | 69mg/kg (69mg/kg) | SENSE ORGANS AND SPECIAL SENSES: "RETINAL CHANGES (PIGMENTARY DEPOSITIONS, RETINITIS, OTHER): EYE" | Annals of Internal Medicine. Vol. 114, Pg. 297, 1991. |
| mouse | LD50 | intravenous | > 70mg/kg (70mg/kg) | United States Patent Document. Vol. #4804651, | |
| mouse | LD50 | oral | 3062mg/kg (3062mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Fundamental and Applied Toxicology. Vol. 32, Pg. 129, 1996. |
| mouse | LD50 | unreported | > 750mg/kg (750mg/kg) | United States Patent Document. Vol. #4857511, | |
| rat | LD50 | intravenous | > 70mg/kg (70mg/kg) | United States Patent Document. Vol. #4804651, | |
| rat | LD50 | oral | 3084mg/kg (3084mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Fundamental and Applied Toxicology. Vol. 32, Pg. 129, 1996. |
| rat | LD50 | unreported | > 750mg/kg (750mg/kg) | United States Patent Document. Vol. #4857511, | |
| women | TDLo | oral | 1gm/kg/6W-I (1000mg/kg) | SKIN AND APPENDAGES (SKIN): NAILS: OTHER | Annals of Internal Medicine. Vol. 107, Pg. 350, 1987. |
Related Products
- Zidovudine
- 3052-28-6
- 30523-88-7
- 3052-50-4
- 30525-89-4
- 3052-61-7
- 30529-70-5
- 30530-42-8
- 30530-43-9
- 3053-17-6
- 30532-36-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View