-
Name
ZINC CYANIDE
- EINECS 209-162-9
- CAS No. 557-21-1
- Article Data62
- CAS DataBase
- Density 1.85 g/cm3
- Solubility Soluble in alkalies, potassium cyanide and ammonia. Insoluble in water and most solvents.
- Melting Point 800 °C
- Formula C2N2Zn
- Boiling Point 25.7 °C at 760 mmHg
- Molecular Weight 117.425
- Flash Point
- Transport Information UN 1713
- Appearance white to off-white powder
- Safety 7-61-60-45-29-28
- Risk Codes 50/53-32-26/27/28
-
Molecular Structure
-
Hazard Symbols
 N,
N, T+
T+
- Synonyms Zinccyanide (6CI,7CI,8CI);Zinc dicyanide;
- PSA 47.58000
- LogP 0.03106
Synthetic route
-
-
773837-37-9
sodium cyanide

-
A
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| In water compds. dissolved in degassed H2O at 80°C, allowed to stand for 1d at 4°C; NaF filtered off, washed with ice-cold EtOH, aq. EtOH mixt. evapd.; | A 78% B n/a |


-
A
-
557-21-1
zinc(II) cyanide

-
D
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

| Conditions | Yield |
|---|---|
| With air In neat (no solvent) sample heating at 4 K/min in air to 300°C, temp. keeping for 3 h; XRD; | A n/a B 1% C n/a D 1% |


| Conditions | Yield |
|---|---|
| slow react. of liq. (CN)2 with Zn at higher temp. up to 115°C; deposition of paracyan layer on Zn is stopping react.; O2 is inhibiting react., benzene vapor and CO2 have no influence on react.;; | |
| Kinetics; at 22°C very slow, studies on influence of temp.; inhibited by O2; | |
| In neat (no solvent) formation on Zn-surface in the cold after several days, at 100 °C after 3 - 4 h;; |

-
A
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With zinc(II) sulfate byproducts: Na2SO4; calcd. amts. of ZnSO4; | |
| With ZnSO4 byproducts: Na2SO4; calcd. amts. of ZnSO4; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: N2; at 550°C, although evolving of N2 but no formation of cyanamide;; | |
| pptn.;; | |
| In neat (no solvent) byproducts: N2; at 550°C, although evolving of N2 but no formation of cyanamide;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| by heating in sealed tube at 300°C; | |
| by heating in sealed tube at 300°C; |


| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| In water passing HCN into a sludge of ZnO;; | |
| In water passing HCN into a sludge of ZnO;; |

| Conditions | Yield |
|---|---|
| In acetic acid on pptg. of soln. of Zn(OH)2 in acetic acid with HCN and drying at 110°C;; |

| Conditions | Yield |
|---|---|
| With KOC(O)CH3 In water passing HCN into an aq. soln. of ZnSO4 and K acetate;; | |
| With KOC(O)CH3 In water passing HCN into an aq. soln. of ZnSO4 and K acetate;; |

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether on mixing of soln. of Zn acetate/methanol and HCN/ether;; on drying ppt. at 75°C, then in vacuum over P2O5;; | |
| In water passing HCN into an aq. soln. of Zn acetate; pptn. is incomplete;; pptn.;; | |
| In water stratifying of aq. Zn acetate with aq. HCN;; pptn.;; |

| Conditions | Yield |
|---|---|
| In water layering water on aq. soln. of zinc compds., slow diffusion for 2-3 wk; filtration, washing with water, ethanol, air drying; |

| Conditions | Yield |
|---|---|
| digestion or boiling a sludge of ZnO and a moderately concd. KCN soln.;; | |
| digestion or boiling a sludge of ZnO and a moderately concd. KCN soln.;; |

-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| In water Electrolysis; electolysis of an aq. soln. of Zn(CN)2*2KCN (no excess of KCN);; anodic pptn. of Zn(CN)2;; |

| Conditions | Yield |
|---|---|
| In ammonia | |
| In ammonia NH3 (liquid); |

| Conditions | Yield |
|---|---|
| In not given Electrolysis; Zn(CN)2 formed as layer on Zn anode in KCN soln.;; | |
| In not given Electrolysis; electrolysis of a 4 n KCN soln. with Zn electrodes using a current density of 4.61 A*m^-2; formation on the anode;; |


| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| In water (The Chem. of Cyano Complexes of the Transition Elements, Academic Press: London, 1976, p. 287); aq. soln. of NaCN was stirred into aq. soln. ofZn compd.; filtered; washed (water); washed (acetone); dried (vac.); |

| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| slow addn. of a moderately concd. soln. of KCN to a soln. of ZnSO4 until the end of pptn.;; washing of the pptn. with water; drying with alc. and diethyl ether;; | |
| slow addn. of a moderately concd. soln. of KCN to a soln. of ZnSO4 until the end of pptn.;; washing of the pptn. with water; drying with alc. and diethyl ether;; |

-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| In water strong acids;; pptn.;; |

| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

| Conditions | Yield |
|---|---|
| pptn.;; | |
| pptn.;; |

-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With zinc ambient temp., CO2 stream; | |
| With Zn ambient temp., CO2 stream; |

| Conditions | Yield |
|---|---|
| With zinc In ammonia fast reaction; | |
| With Zn In ammonia NH3 (liquid); fast reaction; |
-
-
557-21-1
zinc(II) cyanide

-
-
66107-30-0
4-bromophenyl trifluoromethanesulfonate

-
-
623-00-7
4-bromobenzenecarbonitrile

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
66107-30-0
4-bromophenyl trifluoromethanesulfonate

-
-
106-38-7
para-bromotoluene

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
14001-66-2
5-bromo-2-methoxypyrimidine

-
-
38373-47-6
2-methoxypyrimidine-5-carbonitrile

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 115℃; for 3h; Sealed tube; | 100% |
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 85℃; Inert atmosphere; |
-
-
557-21-1
zinc(II) cyanide

-
-
1307213-09-7
(S)-7-bromo-1,2,3,4-tetrahydro-cyclopenta[b]indol-2-ylamine (R)-2-hydroxy-3-phenylpropionic acid salt

-
-
1029691-23-3
(S)-(7-cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With zinc(II) formate; zinc; 1,1'-bis-(diphenylphosphino)ferrocene; palladium In ISOPROPYLAMIDE at 110℃; Product distribution / selectivity; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1303517-13-6
(4a-R,9a-S)-trifluoro-methanesulfonic acid 1-(2,2,2-trifluoro-acetyl)-2,3,4,4a,9,9a-hexahydro-1H-indeno[2,1-b]pyridin-6-yl ester

-
-
1303517-15-8
(4a-R,9a-S)-1-(2,2,2-trifluoro-acetyl)-2,3,4,4a,9,9a-hexahydro-1H-indeno[2,1-b]pyridine-6-carbonitrile

| Conditions | Yield |
|---|---|
| 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 80℃; for 12h; Inert atmosphere; | 100% |
| (diphenylphosphin)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 80℃; for 12h; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1313279-89-8
(R)-tert-butyl 3-(6-chloro-2-(6-fluoroimidazo[1,2-a]pyridin-3-yl)pyrimidin-4-ylamino)piperidine-1-carboxylate

-
-
1313280-19-1
(R)-tert-butyl 3-(6-cyano-2-(6-fluoroimidazo[1,2-a]pyridin-3-yl)pyrimidin-4-ylamino)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 130℃; for 1h; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1323076-87-4
10a-(4-bromophenyl)-2,3,10,10a-tetrahydro-1H,5H-imidazo[1,2-a]pyrrolo[1,2-d]pyrazin-5-one

-
-
1323076-92-1
4-(5-oxo-2,3-dihydro-1H,5H-imidazo[1,2-a]pyrrolo[1,2-d]pyrazin-10a(10H)-yl)benzonitrile

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 150 - 160℃; for 0.666667h; Inert atmosphere; Microwave irradiation; Sealed tube; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1394909-96-6
4-[3-bromo-4-(2,6-dimethyl-4-pyridinyl)-2,5-difluorophenyl]-2,6-dimethyl-(2R,6S)-morpholine

-
-
1394909-97-7
3-[(2R,6S)-2,6-dimethyl-4-morpholinyl]-6-(2,6-dimethyl-4-pyridinyl)-2,5-difluoro-benzonitrile

| Conditions | Yield |
|---|---|
| With triphenylphosphine; tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 150℃; for 18h; Microwave irradiation; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
26163-03-1
2-amino-3-bromo-5-chloropyridine

-
-
869557-28-8
2-amino-5-chloropyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In 1-methyl-pyrrolidin-2-one at 110℃; for 5h; Further stages; | 100% |
| With tetrakis(triphenylphosphine) palladium(0) at 110 - 120℃; for 5h; | 100% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 130℃; for 0.5h; Microwave irradiation; | 100% |
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 130℃; for 0.5h; Microwave irradiation; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 130℃; for 0.5h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; zinc In N,N-dimethyl-formamide at 145℃; for 2.5h; Solvent; Temperature; Microwave irradiation; | 100% |
-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; zinc In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 100% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; zinc In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 100% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; zinc In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 100% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; zinc In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); zinc; XPhos at 95℃; for 2h; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); zinc; XPhos at 95℃; for 1.5h; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
864773-66-0
5-bromo-6-fluoro-3-methyl-1H-indazole

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 100℃; for 16h; Inert atmosphere; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 120℃; for 4h; Inert atmosphere; | |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 120℃; for 4h; Inert atmosphere; |
-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In N,N-dimethyl-formamide at 130℃; for 1h; Inert atmosphere; Microwave irradiation; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1581753-62-9
6-bromo-1-cyclopropyl-3,3-dimethyl-1,3-dihydroindol-2-one

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 85℃; for 16h; Inert atmosphere; | 100% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl-formamide at 110℃; Sealed tube; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); N,N,N,N,-tetramethylethylenediamine; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene at 100℃; for 2h; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
633336-82-0
tert-butyl N-[(4-cyano-2,6-difluorophenyl)methyl]carbamate

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis-(diphenylphosphino)ferrocene In water; N,N-dimethyl-formamide at 120℃; for 1h; | 100% |
-
-
557-21-1
zinc(II) cyanide

-
-
1208110-27-3
C24H26F3NO4S

-
-
1208110-34-2
(2RS)-1'-[2-(2-methylphenyl)ethyl]-1-oxo-3,4-dihydro-1H-spiro[naphthalene-2,2'-piperidine]-6-carbonitrile hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: zinc(II) cyanide; C24H26F3NO4S With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 120℃; for 1.5h; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether; water; ethyl acetate Cooling with ice; | 100% |
-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; zinc In N,N-dimethyl-formamide at 120℃; for 0.5h; Reagent/catalyst; Inert atmosphere; | 100% |
-
-
891785-28-7
6-bromo-isoquinolin-3-ylamine

-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl acetamide at 90℃; for 1h; | 100% |
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl acetamide at 90℃; for 1h; | 0.57 g |
| With tetrakis(triphenylphosphine) palladium(0); zinc In N,N-dimethyl-formamide at 80℃; |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl-formamide at 140℃; for 2.5h; Inert atmosphere; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl-formamide at 140℃; for 2.5h; Inert atmosphere; | 82% |
-
-
557-21-1
zinc(II) cyanide

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 100℃; for 4h; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 100℃; for 4h; Inert atmosphere; | 74% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 100℃; for 3h; Inert atmosphere; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0) In N,N-dimethyl-formamide at 130℃; Inert atmosphere; | 74.1% |
| With tris-(dibenzylideneacetone)dipalladium(0); XPhos In water; N,N-dimethyl-formamide at 150℃; for 1h; Microwave irradiation; | 65.6% |
-
-
557-21-1
zinc(II) cyanide


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); zinc; XPhos In N,N-dimethyl acetamide at 95℃; for 18h; | 100% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl acetamide at 150℃; for 4h; Inert atmosphere; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl acetamide at 150℃; for 4h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); zinc In N,N-dimethyl acetamide at 120℃; for 12h; Inert atmosphere; | 100% |
Zinc cyanide Consensus Reports
Zinc and its compounds, as well as cyanide and its compounds, are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
Zinc cyanide Standards and Recommendations
DOT Classification: 6.1; Label: Poison
OSHA PEL: TWA 5 mg(CN)/m3
ACGIH TLV: CL 5 mg(CN)/m3 (skin)
DFG MAK: 5 mg/m3
NIOSH REL: (Cyanide) CL 5 mg(CN)/m3/10M
Zinc cyanide Specification
This chemical is called Zinc cyanide, and its IUPAC name is zinc dicyanide. With the molecular formula of C2N2Zn, its classification codes are Agricultural Chemical; Experimental pesticide; Unspecified / Unclassified pesticide. The CAS registry number of this chemical is 557-21-1. Additionally, its product categories are Inorganics; Zinc Salts; Metal and Ceramic Science; Salts. Moreover, it's insoluble in cold water, hydrocyanic acid, ethanol, ether and organic acids, slightly soluble in water, soluble in caustic soda, ammonia, acetic acid zinc solution.
Other characteristics of the Zinc cyanide can be summarised as followings: (1)ACD/LogP: -0.25; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.25; (4)ACD/LogD (pH 7.4): -0.25; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 17.42; (8)ACD/KOC (pH 7.4): 17.42; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.79 Å2; (13)Enthalpy of Vaporization: 27.18 kJ/mol; (14)Boiling Point: 25.7 °C at 760 mmHg; (15)Vapour Pressure: 740 mmHg at 25°C.
The Zinc cyanide could be obtained by the reactants of serum zinc chloride and sodium cyanide solution. This reaction needs the processes of separation and drying. The reaction equation is as following: 2NaCN+ZnC12→Zn(CN)2+2NaCl.
Uses of this chemical: The Zinc cyanide is mainly used in electroplating, organic synthesis, medicine and pesticide manufacturing. It could react with 1,3,5-triethyl-benzene to obtain the 2,4,6-triethyl-benzaldehyde. This reaction needs the reagent of HCl , and the yield is about 79 %.
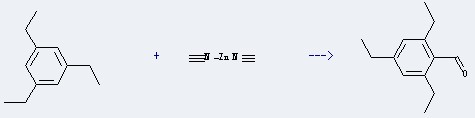
When you are using this chemical, please be cautious about it as the following: This chemical is very toxic by inhalation, in contacting with skin and if swallowed. It's also very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. If contacts with acid, it will liberate very toxic gas. Keep its container tightly closed. Do not empty into drains. Refer to special instructions / safety data sheets if you use it. After contacting with skin, wash immediately with plenty of soap-suds. Moreover, this material and its container must be disposed of as hazardous waste.
You can still convert the following datas into molecular structure:
1.SMILES: [Zn+2].[C-]#N.[C-]#N
2.InChI: InChI=1/2CN.Zn/c2*1-2;/q2*-1;+2
3.InChIKey: GTLDTDOJJJZVBW-UHFFFAOYAM
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LCLo | inhalation | 61mg/m3/4H (61mg/m3) | National Technical Information Service. Vol. OTS0571244, | |
| rat | LD50 | oral | 54mg/kg (54mg/kg) | Toxicologist. Vol. 3, Pg. 170, 1983. | |
| rat | LDLo | intraperitoneal | 100mg/kg (100mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 28, 1953. |
Related Products
- Zinc
- Zinc 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine
- Zinc 2-mercaptobenzothiazole
- zinc 3,5-bis(alpha-methylbenzyl)salicylate
- Zinc acetate
- Zinc acetate dihydrate
- Zinc acetylacetonate hydrate
- Zinc acrylate
- Zinc adenosine triphosphate
- Zinc allyl dithio carbamate
- 55721-11-4
- 55721-31-8
- 55721-65-8
- 55722-32-2
- 55722-49-1
- 55723-45-0
- 557-24-4
- 55724-73-7
- 55725-85-4
- 55726-19-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View