-
Name
tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate
- EINECS 688-142-5
- CAS No. 154026-95-6
- Article Data12
- CAS DataBase
- Density 1.037 g/cm3
- Solubility
- Melting Point
- Formula C15H26O6
- Boiling Point 353.142 °C at 760 mmHg
- Molecular Weight 302.368
- Flash Point 150.334 °C
- Transport Information
- Appearance White or off-white solid powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Rosuvastatin calcium intermediate C-1;(4R-Cis)-6-[(Acetyloxy methyl)]-2,2-Dimethyl-1,3-Dioxane -4-Acetic Acid,1,1-Dimethyethyl Ester;(4R-Cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester(D5);
- PSA 71.06000
- LogP 2.19150
Synthetic route
-
-
10534-59-5
tetrabutylammonium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| at 115℃; for 7h; | 99.1% |
| In 1-methyl-pyrrolidin-2-one at 25 - 95℃; for 24h; | 7.2 g |
-
-
127-09-3
sodium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With copper(l) chloride In N,N-dimethyl-formamide at 120 - 130℃; for 24h; Reagent/catalyst; Solvent; | 97.6% |
| With tetrabutylammomium bromide In N,N-dimethyl-formamide at 120℃; for 4h; Solvent; | 542.97 g |

-
-
77-76-9
2,2-dimethoxy-propane

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In toluene at 20℃; for 8h; | 95.8% |
| With methanesulfonic acid In toluene at 0 - 5℃; for 4h; | 88.1% |
-
-
124655-09-0
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
75-36-5
acetyl chloride

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate With pyridine In dichloromethane at 20℃; for 0.5h; Stage #2: acetyl chloride In dichloromethane at 20℃; for 1h; | 86% |
| With pyridine In dichloromethane at 20℃; for 0.5h; | 15.9 g |
-
-
631-61-8
ammonium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 95℃; for 24h; | 80% |
-
-
127-08-2
potassium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: potassium acetate; (4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester With tetrabutyl phosphonium bromide In toluene at 105℃; for 22h; Stage #2: With pyrographite In n-heptane; toluene at 20℃; | 76% |
| Stage #1: (4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester; 1-ethyl-3-methylimidazolium acetate at 90℃; for 5h; Stage #2: potassium acetate at 90℃; for 23.5h; Product distribution / selectivity; | |
| In 1-ethyl-3-methyl-1H-imidazol-3-ium chloride at 110℃; for 2.5h; |

-
-
144-55-8
sodium hydrogencarbonate

-
-
77-76-9
2,2-dimethoxy-propane

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane; ethyl acetate |


-
-
10534-59-5
tetrabutylammonium acetate

-
-
124655-09-0
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one |

-
-
127-08-2
potassium acetate

-
-
143314-17-4
1-ethyl-3-methylimidazolium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 4h; |

-
-
143314-17-4
1-ethyl-3-methylimidazolium acetate

-
-
154026-94-5
(4R-cis)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-acetic acid,1,1-dimethylethyl ester

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 3h; Product distribution / selectivity; |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / water / 1 h / 0 °C 2.1: sodium tetrahydroborate / water / 14 h / 0 °C 3.1: acetone / 0.25 h / 25 °C 3.2: 6 h / 48 - 50 °C 4.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / water / 1 h / 0 °C 2.1: diethyl methoxy borane / tetrahydrofuran; methanol / 1 h / 25 °C / Inert atmosphere 2.2: 4 h / -78 °C / Inert atmosphere 3.1: acetone / 0.25 h 3.2: 6 h / 48 - 50 °C 4.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / water / 1 h / 0 °C 2.1: sodium tetrahydroborate; sodium dodecyl-sulfate / water / 14 h / 0 °C 3.1: acetone / 0.25 h 3.2: 6 h / 48 - 50 °C 4.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrogenchloride / water / 1 h / 0 °C 2: sodium tetrahydroborate / water / 14 h / 0 °C 3: (R)-10-camphorsulfonic acid / dichloromethane / 3 h / 40 °C 4: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme |
-
-
406680-96-4
(5S)-tert-butyl 6-chloro-3,5-dihydroxyhexanoate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: acetone / 0.25 h / 25 °C 1.2: 6 h / 48 - 50 °C 2.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 2 steps 1: (R)-10-camphorsulfonic acid / dichloromethane / 3 h / 40 °C 2: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme |

-
-
10534-59-5
tetrabutylammonium acetate

-
A
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 25 - 85℃; for 24h; Overall yield = 7.2 %; | A n/a B n/a |


-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water; dichloromethane / 24 h / 20 °C 2: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme |
-
-
154026-92-3
Tert-butyl (5S)-6-chloro-5-hydroxy-3-oxohexanoate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium tetrahydroborate / water / 14 h / 0 °C 2.1: acetone / 0.25 h / 25 °C 2.2: 6 h / 48 - 50 °C 3.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: diethyl methoxy borane / tetrahydrofuran; methanol / 1 h / 25 °C / Inert atmosphere 1.2: 4 h / -78 °C / Inert atmosphere 2.1: acetone / 0.25 h 2.2: 6 h / 48 - 50 °C 3.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium tetrahydroborate; sodium dodecyl-sulfate / water / 14 h / 0 °C 2.1: acetone / 0.25 h 2.2: 6 h / 48 - 50 °C 3.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme |
-
-
154026-93-4
1,1-dimethylethyl (3R,5S)-6-chloro-3,5-dihydroxy-4-hexanoate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: acetone / 0.25 h 1.2: 6 h / 48 - 50 °C 2.1: 1-methyl-pyrrolidin-2-one / 24 h / 25 - 95 °C View Scheme | |
| Multi-step reaction with 2 steps 1: (1S)-10-camphorsulfonic acid / acetone / 6 h / 48 - 50 °C 2: 1-methyl-pyrrolidin-2-one / 24 h / 95 °C View Scheme |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane; water at 25℃; for 48h; | n/a |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: zinc; methanesulfonic acid / tetrahydrofuran / 0.5 h / Reflux 2: D-glucose; potassium dihydrogenphosphate; dipotassium hydrogenphosphate / 25 - 28 °C 3: methanesulfonic acid / toluene / 4 h / 0 - 5 °C View Scheme |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: glucose dehydrogenase; nicotinamide adenine dinucleotide phosphate / N,N-dimethyl-formamide / 25 °C / pH 6.5 / Enzymatic reaction 2: methanesulfonic acid / toluene / 8 h / 20 °C View Scheme |
-
-
74530-56-6
tert-butyl 4-chloroacetoacetate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 6 h / 20 - 60 °C 2.1: ethyl bromide; magnesium; iodine / tetrahydrofuran / 50 - 60 °C 2.2: -10 - 40 °C 3.1: glucose dehydrogenase; nicotinamide adenine dinucleotide phosphate / N,N-dimethyl-formamide / 25 °C / pH 6.5 / Enzymatic reaction 4.1: methanesulfonic acid / toluene / 8 h / 20 °C View Scheme |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: ethyl bromide; magnesium; iodine / tetrahydrofuran / 50 - 60 °C 1.2: -10 - 40 °C 2.1: glucose dehydrogenase; nicotinamide adenine dinucleotide phosphate / N,N-dimethyl-formamide / 25 °C / pH 6.5 / Enzymatic reaction 3.1: methanesulfonic acid / toluene / 8 h / 20 °C View Scheme |

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: zinc; chloro-trimethyl-silane; ethyl bromide / tetrahydrofuran / 60 - 65 °C 1.2: 0 - 40 °C 2.1: glucose dehydrogenase; nicotinamide adenine dinucleotide phosphate / N,N-dimethyl-formamide / 25 °C / pH 6.5 / Enzymatic reaction 3.1: methanesulfonic acid / toluene / 8 h / 20 °C View Scheme |
-
-
74530-57-7
tert-butyl 4-bromoacetoacetate

-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 6 h / 20 - 60 °C 2.1: zinc; chloro-trimethyl-silane; ethyl bromide / tetrahydrofuran / 60 - 65 °C 2.2: 0 - 40 °C 3.1: glucose dehydrogenase; nicotinamide adenine dinucleotide phosphate / N,N-dimethyl-formamide / 25 °C / pH 6.5 / Enzymatic reaction 4.1: methanesulfonic acid / toluene / 8 h / 20 °C View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
154877-92-6
2-((4R,6S)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-yl)acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 60℃; for 20h; | 100% |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
124655-09-0
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; water | 98% |
| With methanol; potassium carbonate for 0.5h; | 98% |
| With methanol; potassium carbonate at 20℃; | 92% |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
124752-23-4
tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

| Conditions | Yield |
|---|---|
| Stage #1: (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester With methanol; potassium carbonate for 3h; Stage #2: With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide In dichloromethane at -5 - 5℃; for 1h; | 96.1% |
| Multi-step reaction with 2 steps 1: potassium carbonate; methanol / 0.5 h 2: copper(l) chloride; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen / acetonitrile; dichloromethane / 25 - 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / dichloromethane; methanol 2: triethylamine; oxalyl dichloride / dichloromethane; dimethyl sulfoxide View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With acetic acid In tetrahydrofuran at 20℃; for 2h; | 90% |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
147489-06-3
(4R,6S)-(E)-{6-[2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-vinyl]-2,2-dimethyl-1,3-dioxan-4-yl}acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; methanol / 0.5 h 2: potassium carbonate / dimethyl sulfoxide / 3 h / 70 °C View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
107-03-9
1-thiopropane

| Conditions | Yield |
|---|---|
| Stage #1: (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester With sodium hydroxide In methanol; water at 15 - 70℃; Stage #2: 1-thiopropane With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at -2 - 2℃; |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / methanol; water / 15 - 70 °C 1.2: -2 - 2 °C 2.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide; sodium hydrogencarbonate; sodium hypochlorite / dichloromethane / -2 - 35 °C View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
135054-68-1
(3R,5S)-6-<(methylsulfonyl)oxy>-3,5-O-isopropylidene-3,5-dihydroxyhexanoic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / methanol / -2 - 2 °C 2: triethylamine / dichloromethane View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
380460-39-9
[(4R,6S)-2,2-dimethyl-6-(1-phenyl-1H-tetrazol-5-ylsulfanylmethyl)[1,3]dioxan-4-yl]acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / methanol / -2 - 2 °C 2: triethylamine / dichloromethane 3: N,N-dimethyl-formamide / 25 - 130 °C View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
380460-37-7
[(4R,6S)-2,2-dimethyl-6-(1-phenyl-1H-tetrazole-5-sulfonylmethyl)[1,3]dioxan-4-yl]acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium carbonate / methanol / -2 - 2 °C 2: triethylamine / dichloromethane 3: N,N-dimethyl-formamide / 25 - 130 °C 4: hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / isopropyl alcohol View Scheme |
-
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
-
289042-12-2
2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / methanol / -2 - 2 °C 2: triethylamine / dichloromethane 3: N,N-dimethyl-formamide / 25 - 130 °C 4: hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / isopropyl alcohol 5: sodium hydride / tetrahydrofuran / 0 - 35 °C View Scheme |
tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate Chemical Properties
Empirical Formula of tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate (CAS NO.154026-95-6): C15H26O6
Molecular Weight: 302.3633
Nominal Mass: 302 Da
Average Mass: 302.3633 Da
Monoisotopic Mass: 302.172939 Da
Index of Refraction: 1.436
Molar Refractivity: 76.312 cm3
Molar Volume: 291.645 cm3
Surface Tension: 33.982 dyne/cm
Density: 1.037 g/cm3
Flash Point: 150.334 °C
Enthalpy of Vaporization: 59.804 kJ/mol
Boiling Point: 353.142 °C at 760 mmHg
Structure of tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate (CAS NO.154026-95-6):
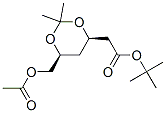
Systematic Name: tert-Butyl 6-O-acetyl-2,4-dideoxy-3,5-O-(1-methylethylidene)-D-erythro-hexonate
SMILES: O=C(OC(C)(C)C)C[C@@H]1OC(O[C@@H](C1)COC(=O)C)(C)C
InChI: InChI=1/C15H26O6/c1-10(16)18-9-12-7-11(19-15(5,6)20-12)8-13(17)21-14(2,3)4/h11-12H,7-9H2,1-6H3/t11-,12+/m1/s1
InChIKey: NGABCYSYENPREI-NEPJUHHUBW
Std. InChI: InChI=1S/C15H26O6/c1-10(16)18-9-12-7-11(19-15(5,6)20-12)8-13(17)21-14(2,3)4/h11-12H,7-9H2,1-6H3/t11-,12+/m1/s1
Std. InChIKey: NGABCYSYENPREI-NEPJUHHUSA-N
tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate Specification
tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate (CAS NO.154026-95-6) also can be called (4R-cis)-6-[(Acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester .
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1-formylcyclopropyl)carbamate
- tert-Butyl (1-hydroxy-3-phenylpropan-2-yl)carbamate
- tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate
- tert-Butyl (2-aminophenyl)carbamate
- tert-Butyl (2-chloro-3-formylpyridin-4-yl)carbamate
- tert-Butyl (2R)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- tert-Butyl (2S)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2S)-2-carbamoyl-2,3-dihydropyrrole-1-carboxylate
- 154027-26-6
- 15402-79-6
- 15402-84-3
- 1540-29-0
- 1540-32-5
- 1540-34-7
- 154044-13-0
- 154048-89-2
- 154051-94-2
- 154057-56-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View