-
Name
tert-Butyl cyanoacetate
- EINECS 214-243-7
- CAS No. 1116-98-9
- Article Data23
- CAS DataBase
- Density 1.001 g/cm3
- Solubility
- Melting Point 107-108oC
- Formula C7H11NO2
- Boiling Point 194.6 °C at 760 mmHg
- Molecular Weight 141.17
- Flash Point 79 °C
- Transport Information
- Appearance colorless liquid
- Safety 24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Aceticacid, cyano-, 1,1-dimethylethyl ester (9CI);Acetic acid, cyano-, tert-butylester (6CI,7CI,8CI);1,1-Dimethylethyl cyanoacetate;Cyanoacetic acidtert-butyl ester;Cyanoacetic acid tert-butyl ester;NSC 1072;tert-Butylcyanoacetate;
- PSA 50.09000
- LogP 1.24178
Synthetic route

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran at 10℃; under 12001.2 - 14251.4 Torr; for 0.133333h; Temperature; Solvent; | 98% |
| With boron trifluoride diethyl etherate In dichloromethane at 10℃; for 7h; Reagent/catalyst; Temperature; Solvent; Large scale; | 90% |
| With sulfuric acid; tert-butyl alcohol at -20℃; |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 5h; Reagent/catalyst; Temperature; Inert atmosphere; | 86% |
| With dicyclohexyl-carbodiimide In acetonitrile at 25℃; for 5h; | 84% |
| With dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 0.25h; | 75% |
-
-
107-59-5
tert-Butyl chloroacetate

-
-
151-50-8
potassium cyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium iodide; tert-butyl alcohol |
-
-
107-59-5
tert-Butyl chloroacetate

-
-
151-50-8
potassium cyanide

-
A
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
B
-
106271-05-0
cyano-succinic acid di-tert-butyl ester

| Conditions | Yield |
|---|---|
| With water; tert-butyl alcohol |

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
151-50-8
potassium cyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With methanol |
-
-
16130-58-8
cyanoacetic acid chloride

-
-
75-65-0
tert-butyl alcohol

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With diethyl ether; N,N-dimethyl-aniline |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
13435-20-6
tetraethylammoniumcyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 50℃; | 100% |
-
-
108-86-1
bromobenzene

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
82102-37-2
9-methyl-9H-fluorene-9-carbonyl chloride

-
-
614-16-4
Benzoylacetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene; cyanoacetic acid tert-butyl ester; 9-methyl-9H-fluorene-9-carbonyl chloride With dichloro(1,5-cyclooctadiene)palladium(II); N-Methyldicyclohexylamine; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; magnesium chloride; tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 100℃; for 18h; Glovebox; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane Reagent/catalyst; Inert atmosphere; | 100% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one for 16h; | 100% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
108-94-1
cyclohexanone

-
-
92932-02-0
2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With morpholine; sulfur In ethanol at 50℃; | 99% |
| With sulfur; diethylamine In ethanol Gewald cyclization; Reflux; | 95% |
| With morpholine; sulfur In ethanol at 45℃; for 5h; | 89% |
-
-
110-91-8
morpholine

-
-
1193-20-0
2-methyltetrahydro-4H-pyran-4-one

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
1373497-01-8
tert-butyl 2-amino-5-methyl-5,7-dihydro-4H-thieno[2,3-c]pyran-3-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogen sulfide In ethanol; ethyl acetate | 99% |

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester With sodium hydride In 1,4-dioxane at 20℃; for 1h; Stage #2: (6-chloropyridazin-3-yl)-[2-(indan-2-ylamino)-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-6-yl]methanone In 1,4-dioxane at 100℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran at 10℃; under 12001.2 - 14251.4 Torr; for 0.133333h; Temperature; Solvent; | 98% |
| With boron trifluoride diethyl etherate In dichloromethane at 10℃; for 7h; Reagent/catalyst; Temperature; Solvent; Large scale; | 90% |
| With sulfuric acid; tert-butyl alcohol at -20℃; |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 5h; Reagent/catalyst; Temperature; Inert atmosphere; | 86% |
| With dicyclohexyl-carbodiimide In acetonitrile at 25℃; for 5h; | 84% |
| With dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 0.25h; | 75% |
-
-
107-59-5
tert-Butyl chloroacetate

-
-
151-50-8
potassium cyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium iodide; tert-butyl alcohol |
-
-
107-59-5
tert-Butyl chloroacetate

-
-
151-50-8
potassium cyanide

-
A
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
B
-
106271-05-0
cyano-succinic acid di-tert-butyl ester

| Conditions | Yield |
|---|---|
| With water; tert-butyl alcohol |

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
151-50-8
potassium cyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With methanol |
-
-
16130-58-8
cyanoacetic acid chloride

-
-
75-65-0
tert-butyl alcohol

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With diethyl ether; N,N-dimethyl-aniline |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
13435-20-6
tetraethylammoniumcyanide

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 50℃; | 100% |
-
-
108-86-1
bromobenzene

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
82102-37-2
9-methyl-9H-fluorene-9-carbonyl chloride

-
-
614-16-4
Benzoylacetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene; cyanoacetic acid tert-butyl ester; 9-methyl-9H-fluorene-9-carbonyl chloride With dichloro(1,5-cyclooctadiene)palladium(II); N-Methyldicyclohexylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; magnesium chloride; tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 100℃; for 18h; Glovebox; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane Reagent/catalyst; Inert atmosphere; | 100% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one for 16h; | 100% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
108-94-1
cyclohexanone

-
-
92932-02-0
2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With morpholine; sulfur In ethanol at 50℃; | 99% |
| With sulfur; diethylamine In ethanol Gewald cyclization; Reflux; | 95% |
| With morpholine; sulfur In ethanol at 45℃; for 5h; | 89% |
-
-
110-91-8
morpholine

-
-
1193-20-0
2-methyltetrahydro-4H-pyran-4-one

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
1373497-01-8
tert-butyl 2-amino-5-methyl-5,7-dihydro-4H-thieno[2,3-c]pyran-3-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogen sulfide In ethanol; ethyl acetate | 99% |

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester With sodium hydride In 1,4-dioxane at 20℃; for 1h; Stage #2: (6-chloropyridazin-3-yl)-[2-(indan-2-ylamino)-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-6-yl]methanone In 1,4-dioxane at 100℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester; 3 -bromo-6-chloro-2-methoxypyridine With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium t-butanolate In 1,4-dioxane at 75℃; for 3.91667h; Inert atmosphere; Stage #2: methyl iodide In 1,4-dioxane at 20℃; | 99% |
| Stage #1: cyanoacetic acid tert-butyl ester; 3 -bromo-6-chloro-2-methoxypyridine With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium t-butanolate In 1,4-dioxane at 75℃; for 3.91667h; Inert atmosphere; Stage #2: methyl iodide In 1,4-dioxane at 18 - 25℃; |


| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester; C15H18N2O2 With C39H38N3O2P; potassium carbonate; silver(l) oxide at 20℃; for 24h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene for 6h; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester; C13H12N2O4 With C39H38N3O2P; potassium carbonate; silver(l) oxide In toluene at 0℃; for 24h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene for 6h; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With C39H38N3O2P; potassium carbonate; silver(l) oxide at 20℃; enantioselective reaction; | 99% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
790-41-0
4-chlorobenzoic anhydride

-
-
724767-52-6
tert-butyl 2-(4-chlorophenyl)-2-cyanoacetate

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -45℃; for 0.75h; Inert atmosphere; Stage #2: 4-chlorobenzoic anhydride In tetrahydrofuran; hexane at -45℃; for 1h; Inert atmosphere; | 98% |
| Stage #1: cyanoacetic acid tert-butyl ester With lithium diisopropyl amide In tetrahydrofuran; hexane at -45℃; for 0.75h; Stage #2: 4-chlorobenzoic anhydride In tetrahydrofuran; hexane at -45 - 20℃; Further stages.; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
32779-36-5
5-Bromo-2-chloropyrimidine

-
-
1305329-97-8
tert-butyl 2-(5-bromopyrimidin-2-yl)-2-cyanoacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 5-Bromo-2-chloropyrimidine In tetrahydrofuran at 20℃; for 18h; | 98% |
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 80℃; for 3h; | 35.72% |
-
-
99368-67-9
2-chloro-5-nitro-3-(trifluoromethyl)pyridine

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 50℃; for 10h; | 98% |
| With potassium carbonate In tetrahydrofuran at 50℃; for 10h; Temperature; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,5,6-tetrafluoropyrimidine With potassium carbonate In acetonitrile Stage #2: cyanoacetic acid tert-butyl ester In acetonitrile at 20℃; for 72h; | 98% |

| Conditions | Yield |
|---|---|
| With C39H38N3O2P; potassium carbonate; silver(l) oxide at 20℃; Reagent/catalyst; enantioselective reaction; | 98% |
-
-
3612-20-2
1-phenylmethyl-4-piperidone

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
872530-68-2
tert-butyl 2-amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate

| Conditions | Yield |
|---|---|
| With morpholine; sulfur at 20℃; for 48h; Gewald Aminoheterocycles Synthesis; | 97% |
| With morpholine; sulfur In ethanol at 45℃; for 5h; | 86% |
| With morpholine; sulfur In ethanol at 50℃; for 2h; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
75629-56-0
1-ethoxycarbonylmethyl-3-(1-pyrrolidinylmethylene)-3H-indolium bromide

-
-
866601-48-1
2-cyano-3-(1-ethoxycarbonylmethyl-1H-indol-3-yl)-acrylic acid

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol; chloroform at 20℃; | 97% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
502-42-1
cycloheptanone

-
-
885115-88-8
tert-butyl 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate

| Conditions | Yield |
|---|---|
| With morpholine; sulfur In ethanol at 45℃; | 97% |
| With morpholine; sulfur In ethanol for 16h; Gewald Aminoheterocycles Synthesis; Reflux; | 64% |
| With morpholine; sulfur In ethanol at 45℃; for 24h; Gewald Aminoheterocycles Synthesis; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
93-97-0
benzoic acid anhydride

-
-
115549-32-1
2-t-butoxycarbonyl-2-phenylacetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid tert-butyl ester With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -45℃; for 0.75h; Inert atmosphere; Stage #2: benzoic acid anhydride In tetrahydrofuran; hexane at -45℃; for 1h; Inert atmosphere; | 97% |
| Stage #1: cyanoacetic acid tert-butyl ester With lithium diisopropyl amide In tetrahydrofuran; hexane at -45℃; for 0.75h; Stage #2: benzoic acid anhydride In tetrahydrofuran; hexane at -45 - 20℃; Further stages.; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
1236004-44-6
tert-butyl α-cyano-α-diazoacetate

| Conditions | Yield |
|---|---|
| With perfluorobutanesulfonyl azide; triethylamine In dichloromethane at 20℃; Inert atmosphere; | 97% |
| With sodium azide; 3-Carboxybenzenesulfonyl chloride; potassium carbonate In water at 20℃; for 1h; | 71% |
| With pyridine; triflic azide In hexane; acetonitrile for 24h; Inert atmosphere; | 69% |
| With pyridine; triflic azide In hexane; acetonitrile at 20℃; for 24h; Inert atmosphere; | |
| Stage #1: cyanoacetic acid tert-butyl ester With potassium carbonate; Methanesulfonyl azide In water at 20℃; Stage #2: With water; potassium carbonate at 20℃; for 3h; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
113583-35-0
4,6-dimethoxypyrimidin-2-yl methyl sulfone

-
-
1225226-84-5
tert-butyl 2-cyano-2-(4,6-dimethoxypyrimidin-2-yl)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60 - 70℃; for 6h; Inert atmosphere; | 97% |
| With potassium carbonate In acetonitrile for 24h; Reflux; | 87.3% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate In dimethyl sulfoxide at 80℃; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 97% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate In dimethyl sulfoxide at 80℃; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With piperidine In tetrahydrofuran | 96% |
-
-
108-86-1
bromobenzene

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
1072315-89-9
9-methylfluorene-9-([(13)C]-carbonyl) chloride

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene; cyanoacetic acid tert-butyl ester; 9-methylfluorene-9-([(13)C]-carbonyl) chloride With dichloro(1,5-cyclooctadiene)palladium(II); N-Methyldicyclohexylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; magnesium chloride; tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 100℃; for 18h; Glovebox; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane Inert atmosphere; | 96% |

-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
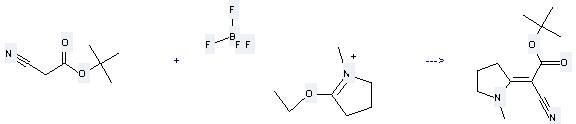
41948-92-9
5-ethoxy-1-methyl-3,4-dihydro-2H-pyrrolium; tetrafluoroborate

-
-
125138-99-0
Cyano-[1-methyl-pyrrolidin-(2E)-ylidene]-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate In tetrahydrofuran for 1h; Ambient temperature; | 95% |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
86194-06-1
C20H14NO(1+)*I3(1-)

-
-
121030-45-3
Cyano-[1-oxo-2,3-diphenyl-1H-indolizin-(7E)-ylidene]-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine for 1h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With C8H18NO3(1+)*C2H4NO2(1-) In water at 20℃; Knoevenagel Condensation; | 95% |
| With piperidine In dichloromethane at 20℃; for 20h; Knoevenagel Condensation; Inert atmosphere; | 71% |
| With piperidine In ethanol Heating; | |
| With piperidine In dichloromethane at 20℃; Inert atmosphere; |
-
-
1116-98-9
cyanoacetic acid tert-butyl ester

-
-
100-52-7
benzaldehyde

-
-
1145-55-7
α-Cyanzimtsaeure-tert-butylester

| Conditions | Yield |
|---|---|
| quaternary ammonium-ordered porous silicate composite In benzene at 80℃; for 6h; Knoevenagel condensation; | 95% |
| With piperidine In ethanol at 70℃; for 19h; | 76% |
| With C21H13N3O6(2-)*Zn(2+)*2H2O In tetrahydrofuran at 50℃; for 1.5h; Knoevenagel Condensation; | 45% |
tert-Butyl cyanoacetate Specification
The Acetic acid, 2-cyano-,1,1-dimethylethyl ester, with the CAS registry number 1116-98-9, is also known as 1,1-Dimethylethyl cyanoacetate. It belongs to the product categories of C6 to C7; Carbonyl Compounds; Esters. Its EINECS number is 214-243-7. This chemical's molecular formula is C7H11NO2 and molecular weight is 141.17. What's more, its IUPAC name is methyl tert-butyl 2-cyanoacetate. It is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Its storage temperature is 2 - 8 °C. What's more, it should be protected from oxides and acids. It is harmful if swallowed. When using it, you should avoid contacting with skin and eyes.
Physical properties of Acetic acid, 2-cyano-,1,1-dimethylethyl ester are: (1)ACD/LogP: 0.76; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.32; (4)ACD/LogD (pH 7.4): -1.73; (5)ACD/BCF (pH 5.5): 1; (6)ACD/KOC (pH 5.5): 1; (7)#H bond acceptors: 3; (8)#H bond donors: 0; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 50.09 Å2; (11)Index of Refraction: 1.426; (12)Molar Refractivity: 36.13 cm3; (13)Molar Volume: 140.9 cm3; (14)Surface Tension: 33.3 dyne/cm; (15)Density: 1.001 g/cm3; (16)Flash Point: 79 °C; (17)Enthalpy of Vaporization: 43.08 kJ/mol; (18)Boiling Point: 194.6 °C at 760 mmHg; (19)Vapour Pressure: 0.438 mmHg at 25°C.
Uses of Acetic acid, 2-cyano-,1,1-dimethylethyl ester: it can be used to produce cyano-(1-methyl-pyrrolidin-2-ylidene)-acetic acid tert-butyl ester at the ambient temperature. It will need reagent NaOMe and solvent tetrahydrofuran with the reaction time of 1 hour. The yield is about 95%.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(C)(C)OC(=O)CC#N
(2)InChI: InChI=1S/C7H11NO2/c1-7(2,3)10-6(9)4-5-8/h4H2,1-3H3
(3)InChIKey: BFNYNEMRWHFIMR-UHFFFAOYSA-N
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (2-aminophenyl)carbamate
- tert-Butyl (3-methylazetidin-3-yl)methylcarbamate
- tert-Butyl (3S)-3-(hydroxymethyl)morpholine-4-carboxylate
- tert-Butyl (3S)-3-amino-4-phenylbutanoate
- tert-Butyl (3S,4R)-4-(2-methoxyphenyl)pyrrolidin-3-ylcarbamate
- tert-Butyl (3S,4R)-4-phenylpyrrolidin-3-ylcarbamate
- tert-Butyl (4-aminophenyl)carbamate
- tert-Butyl (4-bromothiazol-2-yl)methylcarbamate
- tert-Butyl (4-chloropyridin-2-yl)carbamate
- 111-70-6
- 111711-51-4
- 111714-47-7
- 111-71-7
- 111721-75-6
- 1117-31-3
- 111732-86-6
- 1117-37-9
- 111743-55-6
- 111744-92-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View