-
Name
tert-Butyl methacrylate
- EINECS 209-548-7
- CAS No. 585-07-9
- Article Data25
- CAS DataBase
- Density 0.875 g/cm3
- Solubility insoluble in water
- Melting Point -60 °C
- Formula C8H14O2
- Boiling Point 156.9 °C at 760 mmHg
- Molecular Weight 142.198
- Flash Point 41.1 °C
- Transport Information UN 3272
- Appearance colorless liquid with an ester like odor
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms Methacrylicacid, tert-butyl ester (6CI,7CI,8CI);1,1-Dimethylethyl methacrylate;AcryesterTB;Acryester TBMA;Light Ester TB;NSC 20957;tert-Butyl 2-methylacrylate;tert-Butyl 2-methylpropenoate;tert-Butyl alcohol methacrylate;tert-Butylmethacrylate;tert-Butyl a-methacrylate;
- PSA 26.30000
- LogP 1.90420
Synthetic route
-
-
23877-12-5
tert-butyl bromoisobutyrate

-
-
67828-69-7
methyl 5-chloro-2,4-dihydroxybenzoate

-
A
-
1003567-14-3
4-(1-tert-butoxycarbonyl-1-methylethoxy)-5-chloro-2-hydroxybenzoic acid methyl ester

-
B
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl bromoisobutyrate; methyl 5-chloro-2,4-dihydroxybenzoate With tetrabutylammomium bromide; potassium carbonate In 1-methyl-pyrrolidin-2-one at 60 - 65℃; for 16h; Stage #2: With hydrogenchloride In 1-methyl-pyrrolidin-2-one; water pH=~ 4; | A 72.2% B n/a |
| Stage #1: tert-butyl bromoisobutyrate; methyl 5-chloro-2,4-dihydroxybenzoate With potassium carbonate; tetrabutylammomium bromide In 1-methyl-pyrrolidin-2-one at 60 - 65℃; for 16h; Stage #2: With hydrogenchloride In 1-methyl-pyrrolidin-2-one; water pH=~ 4; | A 72.2% B n/a |
| With tetrabutylammomium bromide; potassium carbonate In 1-methyl-pyrrolidin-2-one at 60 - 65℃; for 16h; | A 72.2% B n/a |


| Conditions | Yield |
|---|---|
| With Petroleum ether; tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With pyridine | |
| With pyridine |

| Conditions | Yield |
|---|---|
| With diethyl ether; hydroquinone |
-
-
463-49-0
1,2-propanediene

-
-
201230-82-2
carbon monoxide

-
-
75-65-0
tert-butyl alcohol

-
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| dodecacarbonyl-triangulo-triruthenium at 80℃; under 22800 Torr; for 5h; | 36 % Chromat. |


| Conditions | Yield |
|---|---|
| With pentamethylbenzene, at 19.85℃; Kinetics; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-methylpropenal With tert-butylhypochlorite In tetrachloromethane at 20 - 50℃; for 5.5 - 6.5h; Stage #2: tert-butyl alcohol With triethylamine In tetrachloromethane at 0 - 20℃; for 19.5h; |

-
A
-
585-07-9
tert-Butyl methacrylate

-
B
-
762-04-9, 123-22-8
Diethyl phosphonate

-
C
-
1431319-34-4
C18H38NO6P

-
D
-
188526-94-5
N-(2-methyl-2-propyl)-N-(1-diethylphosphono-2,2-dimethylpropyl)aminoxyl

| Conditions | Yield |
|---|---|
| under 7.50075E-06 Torr; Kinetics; |


| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With tempol; 2,4-dimethyl-6-tert-butylphenol; 4-methoxy-phenol; magnesium bromide at 90℃; for 6h; Reagent/catalyst; |
-
-
76849-54-2
t-butyl pyruvate

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 2h; Stage #2: t-butyl pyruvate In tetrahydrofuran; mineral oil at 0 - 20℃; |
-
-
585-07-9
tert-Butyl methacrylate

-
-
495417-67-9
2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate

- block-copoly{2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate/tert-butyl methacrylate}, anionic polymerization, Mn = 18E3, Mw/Mn = 1.03; Monomer(s): 2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate; tert-butyl methacrylate
-
block-copoly{2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate/tert-butyl methacrylate}, anionic polymerization, Mn = 18E3, Mw/Mn = 1.03; Monomer(s): 2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate; tert-butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: 2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethyl methacrylate With diethylzinc; (diphenylmethyl)potassium In tetrahydrofuran at -78℃; for 2h; Stage #2: tert-Butyl methacrylate In tetrahydrofuran at -78℃; for 1h; | 100% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
495417-68-0
2-[2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate

- block-copoly{2-[2-[2-[(t-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate/tert-butyl methacrylate}, Mn = 9.0E3, Mw/Mn = 1.03; Monomer(s): 2-[2-[2-[(t-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate; tert-butyl methacrylate
-
block-copoly{2-[2-[2-[(t-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate/tert-butyl methacrylate}, Mn = 9.0E3, Mw/Mn = 1.03; Monomer(s): 2-[2-[2-[(t-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate; tert-butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: 2-[2-[2-[(tert-butyldimethylsilyl)oxy]ethoxy]ethoxy]ethyl methacrylate With diethylzinc; (diphenylmethyl)potassium In tetrahydrofuran at -78℃; for 3h; Stage #2: tert-Butyl methacrylate In tetrahydrofuran at -78℃; for 1h; | 100% |
- poly(methyl methacrylate), 1,1-bis[3-(bromomethyl)phenyl]ethyl-terminated, Mn 1.23E4 Da by SEC, Mn 1.35E4 Da by osmometry, Mw/Mn 1.04; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene
-
poly(methyl methacrylate), 1,1-bis[3-(bromomethyl)phenyl]ethyl-terminated, Mn 1.23E4 Da by SEC, Mn 1.35E4 Da by osmometry, Mw/Mn 1.04; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene

-
-
585-07-9
tert-Butyl methacrylate

- poly[(methyl methacrylate)-b-(tert-butyl methacrylate)], asymmetric star-branched, 3-arm, Mw 3.33E4 Da, Mw/Mn 1.02; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; tert-butyl methacrylate
-
poly[(methyl methacrylate)-b-(tert-butyl methacrylate)], asymmetric star-branched, 3-arm, Mw 3.33E4 Da, Mw/Mn 1.02; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; tert-butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-Butyl methacrylate With (diphenylmethyl)potassium In tetrahydrofuran Stage #2: poly(methyl methacrylate), 1,1-bis[3-(bromomethyl)phenyl]ethyl-terminated, Mn 1.23E4 Da by SEC, Mn 1.35E4 Da by osmometry, Mw/Mn 1.04; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene In tetrahydrofuran at -78℃; for 1h; | 100% |
- poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene
-
poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene

-
-
585-07-9
tert-Butyl methacrylate

- poly[(methyl methacrylate)-b-(tert-butyl methacrylate)], asymmetric star-branched, 5-arm, Mw 6.27E4 Da, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; tert-butyl methacrylate
-
poly[(methyl methacrylate)-b-(tert-butyl methacrylate)], asymmetric star-branched, 5-arm, Mw 6.27E4 Da, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; tert-butyl methacrylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-Butyl methacrylate With (diphenylmethyl)potassium In tetrahydrofuran Stage #2: poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene In tetrahydrofuran at -78℃; for 1h; | 100% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
912809-59-7
3-bromo-2-methyl-acrylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: tert-Butyl methacrylate With bromine In tetrachloromethane at 25℃; for 2h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrachloromethane at 25℃; for 5h; | 100% |
| Multi-step reaction with 2 steps 1: bromine / tetrachloromethane / 0 - 20 °C / Inert atmosphere 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0 - 20℃; for 2h; | 100% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
67832-11-5
4-bromo-2-methylbenzonitrile

-
-
929202-11-9
3-(4-cyano-3-methyl-phenyl)-2-methyl-acrylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); N-Methyldicyclohexylamine; johnphos In 1,4-dioxane at 80℃; Heck coupling; | 100% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
854416-76-5
tert-butyl 2,3-dibromo-2-methylpropanoate

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 0 - 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: tert-Butyl methacrylate; pentan-3-one With potassium tert-butylate In tetrahydrofuran at 25℃; for 0.5h; Michael addition-Claisen condensation; Stage #2: methyl iodide In tetrahydrofuran at 50℃; for 3h; Further stages.; | 98% |

-
-
585-07-9
tert-Butyl methacrylate

-
-
219506-75-9
2-benzyloxycarbonyl-1-butene

| Conditions | Yield |
|---|---|
| With 1,1-Diphenylpropyllithium In tetrahydrofuran at -78℃; for 15h; | 98% |

| Conditions | Yield |
|---|---|
| With Cp*TiCl3; triethylamine hydrochloride; zinc In ethyl acetate at 22℃; for 12h; Inert atmosphere; Glovebox; | 98% |
-
-
563-80-4
3-methyl-butan-2-one

-
-
585-07-9
tert-Butyl methacrylate

-
-
104355-62-6
4,4,6-trimethylcyclohexane-1,3-dione

| Conditions | Yield |
|---|---|
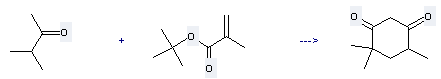
| With potassium tert-butylate In tetrahydrofuran at 25℃; for 0.5h; Michael addition-Claisen condensation; | 97% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
122570-91-6
diethyl (((diphenylmethylene)amino)methyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: diethyl N-(diphenylmethylene) aminomethyl phosphonate With C36H45NO9; sodium t-butanolate In toluene at -78℃; for 0.166667h; Michael Addition; Inert atmosphere; Stage #2: tert-Butyl methacrylate In toluene at -78℃; for 1h; Michael Addition; Inert atmosphere; stereoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-((((benzyloxy)carbonyl)amino)oxy)-2-methylpropanoic acid With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; caesium carbonate In dichloromethane at 25℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: 2-ethyl-1-butene; tert-Butyl methacrylate With water In dichloromethane at 25℃; for 24h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; chemoselective reaction; | 93% |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 1.5h; Michael addition; | 92% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
696-62-8
para-iodoanisole

-
-
1415412-15-5
(E)-tert-butyl 3-(4-methoxyphenyl)-2-methylacrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; for 6h; Heck Reaction; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With manganese; C52H52Cl2F6N2O2Ti; triethylamine hydrochloride In ethyl acetate at 22℃; for 12h; Inert atmosphere; Sealed tube; stereoselective reaction; | 91% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
126688-97-9
2-[(1E)-hex-1-en-1-yl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate In N,N-dimethyl acetamide at 50℃; for 6h; | 90% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
138336-33-1
tert-Butyl 2-(Phenylsulfonyl)acetate

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile Michael Addition; Inert atmosphere; Reflux; | 90% |
-
-
12120-15-9
ethylenebis(triphenylphosphine)platinum(0)

-
-
585-07-9
tert-Butyl methacrylate

-
-
186495-03-4
Pt(P(C6H5)3)2(CH2C(CH3)COOC(CH3)3)

| Conditions | Yield |
|---|---|
| In toluene byproducts: C2H4; N2-atmosphere; stirring equimolar amts. for 2 h; cooling to 0°C, pptn. on addn. of petroleum ether, collection (filtration), washing (petroleum ether), drying (vac.); elem. anal.; | 89% |
-
-
591-50-4
iodobenzene

-
-
585-07-9
tert-Butyl methacrylate

-
-
98831-01-7
(2E)-tert-butyl 2-methyl-3-phenylprop-2-enoate

| Conditions | Yield |
|---|---|
| With potassium carbonate at 80℃; Heck Reaction; Micellar solution; | 89% |
-
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| With ethyl 2-bromoisobutyrate; [(1,3,5-iPr3C6H3)Ru(μ-Cl)3RuCl(C2H4)(PCy3)] In toluene at 35℃; | 88% |
-
-
585-07-9
tert-Butyl methacrylate

| Conditions | Yield |
|---|---|
| With Methyl 2-bromopropionate; N,N,N',N'',N'''-pentamethyldiethylenetriamine; copper(I) bromide; copper(ll) bromide In acetone at 60℃; for 1.33333h; | 86% |
-
-
96-27-5
rac-3-sulfanylpropane-1,2-diol

-
-
585-07-9
tert-Butyl methacrylate

-
-
167768-11-8
6,7-dihydroxy-2-methyl-4-thiaheptanoic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine for 0.5h; Ambient temperature; | 84% |
| With sodium bicarbonate; triethylamine; citric acid In water | 84% |
-
-
585-07-9
tert-Butyl methacrylate

-
-
780-69-8
triethoxyphenylsilane

-
-
70836-95-2, 107772-28-1, 126639-62-1
methyl-3-phenyl-propionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cyclo-octa-1,5-diene; [Rh(OH)(cod)]2 In 1,4-dioxane at 90℃; for 16h; | 84% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; potassium tert-butylate In N,N-dimethyl acetamide at 25℃; under 760.051 Torr; for 12h; Glovebox; Schlenk technique; Irradiation; Sealed tube; regioselective reaction; | 84% |

-
-
585-07-9
tert-Butyl methacrylate

-
-
110269-68-6
tert-butyl ((1R,4R)-bornylideneamino)acetate

-
-
133445-88-2, 133521-71-8
di-tert-butyl (2R)-2-<(1R,4R)-bornylideneamino>-4-methylglutamate

| Conditions | Yield |
|---|---|
| With n-butyllithium; tert-butyl alcohol In tetrahydrofuran; hexane at -78℃; for 18h; | 83% |

-
-
585-07-9
tert-Butyl methacrylate

-
-
136750-17-9
C20N4H8(C6H5)4AlOCOCCH3C2H5CO2C4H9

| Conditions | Yield |
|---|---|
| With carbon dioxide In benzene under irradiation with visible light at 30°C for 3 h;; observed by NMR spectroscopy;; | 83% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20℃; for 48h; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: triethylsilane With 1,3,5-trimethyl-2,4,6-tris-(3,5-di-tert-butyl-4-hydroxybenzyl)benzene; tris(pentafluorophenyl)borate at 20℃; for 0.5h; Neat (no solvent); Stage #2: tert-Butyl methacrylate at 3.5 - 6℃; for 5.5h; Neat (no solvent); Cooling with ice water; | 81.5% |
tert-Butyl methacrylate Specification
The tert-Butyl methacrylate, with the CAS registry number 585-07-9, is also known as 1,1-Dimethylethyl 2-methyl-2-propenoate. It belongs to the product categories of Medical Intermediates; Acrylic Monomers; C8 to C9Monomers; Carbonyl Compounds; Esters; Methacrylate. Its EINECS registry number is 209-548-7. This chemical's molecular formula is C8H14O2 and molecular weight is 142.19556. Its IUPAC name is called tert-butyl 2-methylprop-2-enoate. What's more, this chemical's classification codes are Drug / Therapeutic Agent; TSCA Flag P [A commenced PMN (Premanufacture Notice) substance]. When you are using this chemical, please be cautious about it. It is flammable. You should keep it away from sources of ignition - No smoking.
Physical properties of tert-Butyl methacrylate: (1)ACD/LogP: 2.57; (2)ACD/LogD (pH 5.5): 2.57; (3)ACD/LogD (pH 7.4): 2.57; (4)ACD/BCF (pH 5.5): 53.22; (5)ACD/BCF (pH 7.4): 53.22; (6)ACD/KOC (pH 5.5): 598.63; (7)ACD/KOC (pH 7.4): 598.63; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.422; (11)Molar Refractivity: 40.42 cm3; (12)Molar Volume: 159 cm3; (13)Surface Tension: 24.9 dyne/cm; (14)Density: 0.894 g/cm3; (15)Flash Point: 41.1 °C; (16)Enthalpy of Vaporization: 39.36 kJ/mol; (17)Boiling Point: 156.9 °C at 760 mmHg; (18)Vapour Pressure: 2.82 mmHg at 25°C.
Uses of tert-Butyl methacrylate: it can be used to produce 4,4,6-trimethyl-cyclohexane-1,3-dione with 3-methyl-butan-2-one at temperature of 25 °C. This reaction is a kind of Michael addition-Claisen condensation. It will need reagent t-BuOK and solvent tetrahydrofuran with reaction time of 0.5 hours. The yield is about 97%.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(=C)C(=O)OC(C)(C)C
(2)InChI: InChI=1S/C8H14O2/c1-6(2)7(9)10-8(3,4)5/h1H2,2-5H3
(3)InChIKey: SJMYWORNLPSJQO-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 8549mg/kg (8549mg/kg) | Toxicology Letters. Vol. 11, Pg. 125, 1982. |
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1-formylcyclopropyl)carbamate
- tert-Butyl (1-hydroxy-3-phenylpropan-2-yl)carbamate
- tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate
- tert-Butyl (2-aminophenyl)carbamate
- tert-Butyl (2-chloro-3-formylpyridin-4-yl)carbamate
- tert-Butyl (2R)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- tert-Butyl (2S)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2S)-2-carbamoyl-2,3-dihydropyrrole-1-carboxylate
- 5850-86-2
- 585-09-1
- 5850-93-1
- 58509-59-4
- 58511-20-9
- 58511-63-0
- 58514-96-8
- 58520-03-9
- 58520-04-0
- 58520-45-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View