-
Name
1-Butene
- EINECS 203-449-2
- CAS No. 106-98-9
- Article Data1336
- CAS DataBase
- Density 0.626 g/cm3
- Solubility Insoluble in water, slightly soluble in benzene, soluble in ethanol, ether
- Melting Point -185 °C(lit.)
- Formula C4H8
- Boiling Point - 6.3 °C(lit.)
- Molecular Weight 56.1075
- Flash Point 80 °C
- Transport Information UN 1012 2.1
- Appearance Colorless gas
- Safety 9-16-33
- Risk Codes 12
-
Molecular Structure
-
Hazard Symbols
 F+,
F+,  F
F
- Synonyms 1-Butene;But-1-ene;Butene, all isomers;CCRIS 8970;UNII-LY001N554L;alpha-Butene;alpha-Butylene;
- PSA 0.00000
- LogP 1.58240
Synthetic route
-
-
17486-13-4
(Z)-crotyltrimethylsilane

-
-
106-98-9
1-butylene

| Conditions | Yield |
|---|---|
| With tetrahydrofuran at 60℃; for 3h; | 100% |
-
-
18284-36-1, 27497-56-9
hydridotetakis(triphenylphosphine)rhodium(I)

-
-
702-04-5
crotyl phenyl sulfide

-
A
-
106-98-9
1-butylene

-
B
-
92922-07-1
{Rh(SC6H5)(P(C6H5)3)2}2

-
C
-
107-01-7
butene-2

| Conditions | Yield |
|---|---|
| In toluene byproducts: P(C6H5)3; (N2); at room temp.; GLC yields of organic compounds; | A 80% B 100% C 15% |


| Conditions | Yield |
|---|---|
| With tetrabutoxytitanium; butyl magnesium bromide; triethylaluminum In n-heptane at 25℃; under 6080 Torr; | 99.4% |
| With tetrabutoxytitanium; butyl magnesium bromide; triethylaluminum In n-heptane at 25℃; under 6080 Torr; Product distribution; Kinetics; var. temp., var. time, var. reagents ratio; | 99.4% |
| With triethylaluminum at 200 - 220℃; |

| Conditions | Yield |
|---|---|
| In benzene-d6 Kinetics; thermolysis at 167 +/- 1°C; | A <1 B <1 C 98% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-85-1
ethene

-
E
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (70 h); | A 0.4% B 0.6% C 1.1% D 97.8% E 0.1% |
| In toluene thermal decompn. at 95°C (23 h); | A 8% B 20.9% C 46.3% D 20.2% E 4.6% |


| Conditions | Yield |
|---|---|
| With ReOx/Al2O3; Ni-AlKIT-6 at 60℃; under 22502.3 Torr; Inert atmosphere; | A 97.4% B n/a |
| With aluminosilicate B In water at 600℃; for 3.75h; | A 21.1% B 51.3% |
| With H-SSZ-13 zeolite consisting of chabazite cages connected via 8-ring windows at 399.84℃; under 375.038 Torr; for 0.25h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
109-52-4
valeric acid

-
A
-
106-98-9
1-butylene

-
B
-
72679-69-7
2-butyl-isonicotinonitrile

-
C
-
7136-18-7
3-n-butyl-4-cyanopyridine

-
D
-
74808-77-8
2,5-di-n-butyl-4-cyanopyridine

-
E
-
74825-01-7
2,3-di-n-butyl-4-cyanopyridine

-
F
-
72679-70-0
2,6-di-n-butyl-4-cyanopyridine

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; sulfuric acid; copper diacetate; silver nitrate In water at 57℃; for 3h; Product distribution; Rate constant; Mechanism; without cupric acetate, various temperatures and acidities, (NH4)2S2O8 for K2S2O8; | A n/a B 96.1% C 0.9% D 2% E 0.5% F 0.7% |


| Conditions | Yield |
|---|---|
| With water; oxygen at 25 - 575℃; under 1800.18 - 9000.9 Torr; | 95% |
| With water; hydrogen; oxygen | 95.9% |
| 35% iron on alumina at 690℃; for 3h; Product distribution / selectivity; | |
| With ZSM-5 zeolite at 550℃; under 1125.11 Torr; for 3h; Gas phase; | |
| With propene at 200℃; under 4560.31 Torr; Catalytic behavior; Reagent/catalyst; |
-
-
18284-36-1, 27497-56-9
hydridotetakis(triphenylphosphine)rhodium(I)

-
-
701-75-7
3-(phenylthio)but-1-ene

-
A
-
106-98-9
1-butylene

-
B
-
92922-07-1
{Rh(SC6H5)(P(C6H5)3)2}2

-
C
-
107-01-7
butene-2

| Conditions | Yield |
|---|---|
| In toluene byproducts: P(C6H5)3; (N2); at room temp.; GLC yields of organic compounds; | A 4% B 95% C 78% |

-
-
190260-52-7
(4R)-4-ethyl-2-phenyl-4,5-dihydro-1λ6,3-thiazole 1,1-dioxide

-
A
-
106-98-9
1-butylene

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| at 600℃; under 0.001 Torr; | A 78% B 94% |

| Conditions | Yield |
|---|---|
| Ni(DMPMNBu)Cl2 In toluene at 30 - 60℃; under 15201 Torr; Product distribution / selectivity; Autoclave; Gas phase; | A 93.7% B 5.1% |
| yttrium; nickel(II) at 199.9℃; Product distribution; various Ni-substituted catalysts; | A 7.1% B 90% |
| With C16H19Br2N4NiP In toluene at 30℃; under 6000.6 Torr; for 0.5h; Catalytic behavior; Time; Reagent/catalyst; Temperature; |

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-85-1
ethene

-
E
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (23 h); | A 93.1% B 1.3% C 0.3% D 0.5% E 4.8% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
16971-06-5, 19696-06-1
trans-hydridoiodobis(triethylphosphine)platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 283.66-313.16 K; NMR; | A 91.8% B 5.9% C n/a D 3.1% |


-
A
-
106-98-9
1-butylene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In benzene acidolysis with equimolar amts. of HCl in benzene at room temp.; | A 91% B 77% |

| Conditions | Yield |
|---|---|
| Kinetics; byproducts: CO2; decopose rapidly at room temp.; (1)H-NMR; GC; | A 91% B 9% |

| Conditions | Yield |
|---|---|
| In solid heated at 200°C in the solid state under reduced pressure in a sealed tube; | A 6% B 18% C 91% D 24% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
37809-11-3
trans-bis(triethylphosphine)(hydrido)(selenocyanato) platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; NMR; | A 90.6% B 5.4% C n/a D 4% |


| Conditions | Yield |
|---|---|
| [Ph2PC6H4C(OB(C6F5)3)O-κ2P,O](η3-CH2CMeCH2) In toluene at 0℃; under 2280.15 Torr; for 1h; Product distribution; Further Variations:; Temperatures; Pressures; oligomerization; | A 90% B 6% |
| With tetraphenyl phosphonium chloride; chromium at 80℃; under 37503.8 Torr; for 1h; Product distribution / selectivity; | A 7.5% B 88.3% |
| With [6,6'-diphenyl-[2,2']-bipyridinyl]NiBr2; triethyl aluminum sesquichloride In toluene Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | A 88% B 9% |
-
-
78-92-2, 15892-23-6
iso-butanol

-
-
106-98-9
1-butylene

| Conditions | Yield |
|---|---|
| zirconium(IV) oxide at 220℃; Product distribution; other catalyst; other methyl substituted 2-butanols; | 90% |
| Hf-Zr oxide at 250℃; Product distribution; study of the catalytic conversion of alcohols, influence of the surface composition of the catalyst on the selectivity; | |
| With PPA at 60 - 65℃; for 0.25h; | |
| With silica-supported sodium phosphate at 400℃; Inert atmosphere; |

-
-
106-98-9
1-butylene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 heating for 4 h at 60°C; (1)H-NMR; | 90% |
| In chloroform-d1 heating for 1 h 120°C; GC gas analysis; | 85% |
-
-
5453-44-1
allyl crotonate

-
-
15681-48-8
lithium dimethylcuprate

-
A
-
106-98-9
1-butylene

-
B
-
107-93-7
(E)-but-2-enoic acid

| Conditions | Yield |
|---|---|
| In diethyl ether at -10℃; for 2h; | A n/a B 89% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) 150°C, 25 h; further products; | A 76% B 89% C 4% |


| Conditions | Yield |
|---|---|
| With Rh(I)[PPh2CH2CH2Si(OEt)3]3Cl; triphenylphosphine; isopropyl alcohol In tetrahydrofuran at 25℃; for 6h; Reagent/catalyst; chemoselective reaction; | 89% |

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
106-99-0
buta-1,3-diene

-
E
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (70 h); | A 88.9% B 3.3% C 2.8% D 4.2% E 0.8% |


| Conditions | Yield |
|---|---|
| With sodium iodide; tin(ll) chloride In ethanol for 0.0333333h; Reflux; Green chemistry; | 88% |
| With B single-collision conditions; | |
| With carbon monoxide; C29H32IrN5O; bis(trifluoromethane)sulfonimide lithium In benzene-d6 at 80℃; under 7500.75 Torr; for 24h; Schlenk technique; chemoselective reaction; | 65 %Spectr. |

| Conditions | Yield |
|---|---|
| at 496.9℃; for 0.25h; Product distribution; | A 86% B 2% C n/a |


| Conditions | Yield |
|---|---|
| With hydrogen; acetic acid In water at 39.84℃; for 4h; Inert atmosphere; | A n/a B n/a C 86% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 60°C (15 h); further product: cyclobutane; | A 85.9% B 3.4% C 3.6% D 1% E 5.7% |
| In toluene thermal decompn. at 95°C (15 h); further product: cyclobutane; | A 58.7% B 2.5% C 2.5% D 1% E 36.3% |


| Conditions | Yield |
|---|---|
| aluminum oxide at 350℃; under 1125.11 Torr; for 20h; Conversion of starting material; | 84% |
| molecular sieve Rate constant; rate constants for dehydratation at various temperatures; | |
| With aluminum oxide at 175 - 500℃; |

| Conditions | Yield |
|---|---|
| cation-exchanger at 90℃; for 1.5h; drying by azeotropic distillation, industrial production; | 100% |
| With sulfuric acid at 100℃; under 5148.6 - 25742.8 Torr; und Destillation im Butylenstrom unter Atmosphaerendruck bei ca. 85grad; | |
| With C18H16O3PS(1+)*HO4S(1-) at 90℃; for 4h; | |
| With Fe/Pd metal modified with hydrogen and nitrogen type cation exchange resin at 100℃; under 22502.3 Torr; for 500h; Reagent/catalyst; | |
| With sulfuric acid at 100℃; under 5148.6 - 25742.8 Torr; und Destillation im Butylenstrom unter Atmosphaerendruck bei ca. 85grad; |
-
-
106-98-9
1-butylene

-
-
111470-27-0
5,5-Di-tert-butyl-1-(di-tert-butylchlorsilyl)-4-(tri-tert-butylsilyl)-1,2,3,4-tetraaza-5-sila-2-cyclopenten

-
B
-
69322-38-9
tri-t-butylsilyl azide

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 168h; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: PhCH=CH2; | 100% |
| In dichloromethane byproducts: PhCH=CH2; (Ar); stirring (15 min, room temp.); elem. anal.; | 97% |
-
-
106-98-9
1-butylene

-
-
161891-78-7
Di-tert.-butyl-(di-tert.-butylphenylsilyl)iminosilan

-
-
1231250-52-4
MeHC=CHCH2Si(tBu)2NHSiPh(tBu)2

| Conditions | Yield |
|---|---|
| In hexane at -78℃; for 4h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In 2,2,4-trimethylpentane at -60 - 100℃; for 2h; Temperature; Reagent/catalyst; Solvent; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen; Bi-Mo oxide (1/1) at 400℃; Rate constant; also without O2; | 99% |
| With oxygen Gas phase; | 99.4% |
| With oxygen Flow reactor; Inert atmosphere; | 99.4% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen at 180℃; under 37503.8 Torr; for 2h; Temperature; Pressure; Autoclave; | 99% |
| With pumice stone; nickel at 150 - 200℃; Hydrogenation; | |
| With hydrogen; tetrahydrofuran; samarium at 20.9℃; under 135 Torr; |
-
-
106-98-9
1-butylene

-
-
460-96-8
bis(trifluoromethyl)phosphine

-
-
20608-42-8
n-Butylbis-trifluormethyl-phosphin

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); time of irradiation:1 h; | 99% |
| Irradiation (UV/VIS); time of irradiation:1 h; | 99% |
| Irradiation; |
-
-
106-98-9
1-butylene

-
-
71639-56-0
{(N,N,N',N'-tetramethylethylenediamine)Cl(η2-ethylene)platinum}ClO4

-
-
476337-45-8
[PtCl(η2-1-butene)(N,N,N',N'-tetramethylethylenediamine)]ClO4

| Conditions | Yield |
|---|---|
| In not given Fanizzi, F. P.; Maresca, L.; Natile, G.; Pacifico, C. Gazz. Chim. Ital. 1994, 124, 137; evapn.; elem. anal.; | 99% |
-
-
694-32-6
1-methyl-2-imidazolidone

-
-
106-98-9
1-butylene

-
-
1146944-10-6
1-sec-butyl-3-methylimidazolidin-2-one

| Conditions | Yield |
|---|---|
| With 2-(di-tert-butylphosphino)-1,1'-biphenylgold(I) chloride; silver trifluoromethanesulfonate In 1,4-dioxane at 100℃; under 6205.94 Torr; for 69h; Autoclave; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst at 60℃; for 6h; | 98.8% |
-
-
106-98-9
1-butylene

-
-
80-15-9
Cumene hydroperoxide

-
A
-
106-88-7
ethyloxirane

-
B
-
617-94-7
1-methyl-1-phenylethyl alcohol

| Conditions | Yield |
|---|---|
| With Ti-HMS at 95℃; under 26252.6 Torr; | A 98.6% B n/a |


| Conditions | Yield |
|---|---|
| With dicarbonylacetylacetonato rhodium (I); C41H30O8P2; hydrogen In toluene at 90℃; under 3750.38 - 7500.75 Torr; for 3h; Reagent/catalyst; regioselective reaction; | 98.2% |
| With [bmim][n-C8H17OSO3]; hydrogen; 2,7-bis(SO3Na)-4,5-bis(PPh2)-9,9-Me2-xanthene Rh complex at 120℃; under 7950.8 Torr; for 0.00472222h; Kinetics; Activation energy; Further Variations:; Pressures; Temperatures; syngas composition, educt conc., catalyst conc.; | 97.7% |
| With (acetylacetonato)dicarbonylrhodium (l); C43H53O8P; hydrogen In toluene under 37503.8 Torr; for 12h; Catalytic behavior; Reagent/catalyst; regioselective reaction; | 97.4% |
-
-
106-98-9
1-butylene

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; LiCB11Me12 In 1,2-dichloro-ethane at 25℃; for 18h; UV-irradiation; | 98% |
-
-
106-98-9
1-butylene

-
-
74-85-1
ethene

-
-
292638-85-8
acrylic acid methyl ester

-
-
818-57-5
Methyl 4-pentenoate

| Conditions | Yield |
|---|---|
| In dichloromethane | 98% |

-
-
106-98-9
1-butylene

-
-
74-85-1
ethene

-
-
292638-85-8
acrylic acid methyl ester

-
-
818-57-5
Methyl 4-pentenoate

| Conditions | Yield |
|---|---|
| In dichloromethane | 98% |

-
-
106-98-9
1-butylene

-
-
1423875-77-7
(2,6-bis(4,4-dimethyloxazolinyl)-3,5-dimethylphenyl)Ir(acetate)(H)

| Conditions | Yield |
|---|---|
| With sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate In benzene-d6 at 20℃; under 760.051 Torr; for 0.25h; Inert atmosphere; Glovebox; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfide; 2,2'-azobis-(2,4-dimethylvaleronitrile) In water at 90℃; for 4h; Autoclave; | 97.6% |
| at 20℃; for 24h; Inert atmosphere; Irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate; dibenzoyl peroxide In water at 90℃; for 4h; Autoclave; | 97.5% |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; phosphoric acid tributyl ester; dihydrogen peroxide In toluene at 70℃; under 3750.38 Torr; for 5h; Temperature; Pressure; | 96.8% |
| With tert.-butylhydroperoxide; 2C13H10N3O2(1-)*MoO2(2+) In methanol; dichloromethane for 1h; Catalytic behavior; Reagent/catalyst; | 91% |
| With dihydrogen peroxide; teterabutylammonium In acetonitrile at 31.85℃; for 8h; | 88% |

| Conditions | Yield |
|---|---|
| 96.4% |
-
-
106-98-9
1-butylene

-
-
110-71-4
1,2-dimethoxyethane

-
-
139973-40-3, 125782-19-6
Re(CC(CH3)3)(CHC(CH3)3)(OC(CH3)(CF3)2)2

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; benzene byproducts: neohexene; under N2, drybox or Schlenk techniques; glass bomb with complex in DME and benzene, cooled (-196°C), degassed, 1-butene condensed into vessel, thawed, vessel wrapped with foil, soln. stirred (2.5 h), addn. of DME; evapn.; elem. anal.; | 96% |

α-Butene Specification
The α-Butene with CAS registry number of 106-98-9 is also known as 1-Butene. The IUPAC name is But-1-ene. It belongs to product categories of Gas Cylinders; Hydrocarbons (Low Boiling point); Synthetic Organic Chemistry; Chemical Synthesis; Compressed and Liquefied Gases; Synthetic Reagents. Its EINECS registry number is 203-449-2. In addition, the formula is C4H8 and the molecular weight is 56.11. This chemical is a colorless gas that insoluble in water and has an extremely low flash point or boiling point. Besides, the gases catch fire in contact with air. What's more, it is one important basic chemical raw material that should be sealed in ventilated, cool room away from oxidants.
Physical properties about α-Butene are: (1)ACD/LogP: 2.34; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.337; (4)ACD/LogD (pH 7.4): 2.337; (5)ACD/BCF (pH 5.5): 35.149; (6)ACD/BCF (pH 7.4): 35.149; (7)ACD/KOC (pH 5.5): 444.817; (8)ACD/KOC (pH 7.4): 444.817; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.371; (11)Molar Refractivity: 20.306 cm3; (12)Molar Volume: 89.656 cm3; (13)Surface Tension: 16.354 dyne/cm; (14)Density: 0.626 g/cm3; (15)Enthalpy of Vaporization: 22.07 kJ/mol; (16)Vapour Pressure: 2213.905 mmHg at 25 °C.
Preparation of α-Butene: it is separated from the C4 fractionis. It also can be prepared by reaction of trimethylsilyl-cis-2-buten. The reaction needs reagent THF at the temperature of 60 °C for 3 hours. The yield is about 100%.
![]()
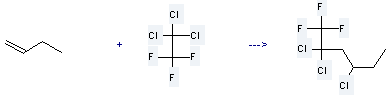
Uses of α-Butene: it is used to produce 2,2,4-trichloro-1,1,1-trifluoro-hexane by reaction with 1,1,1-trichloro-2,2,2-trifluoro-ethane. The reaction occurs with reagents CuCl, CuCl2*H2O, ethanolamine and solvent 2-methyl-propan-2-ol at the temperature of 85 °C for 15 hours. The yield is about 62%.
When you are using this chemical, please be cautious about it. As a chemical, it is extremely flammable. During using it, keep away from sources of ignition and take precautionary measures against static discharges. Furthermore, keep container in a well-ventilated place.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCC=C
2. InChI: InChI=1S/C4H8/c1-3-4-2/h3H,1,4H2,2H3
3. InChIKey: VXNZUUAINFGPBY-UHFFFAOYSA-N
Related Products
- α-Butene
- 106-99-0
- 106990-43-6
- 106996-32-1
- 106997-00-6
- 1070-00-4
- 107001-49-0
- 1070-03-7
- 107007-99-8
- 107008-28-6
- 1070-10-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View