-
Name
(E)-4-Phenyl-3-buten-2-one
- EINECS 217-587-6
- CAS No. 1896-62-4
- Article Data418
- CAS DataBase
- Density 1.014 g/cm3
- Solubility practically insoluble in water
- Melting Point 39-42 °C(lit.)
- Formula C10H10O
- Boiling Point 260.8 °C at 760 mmHg
- Molecular Weight 146.189
- Flash Point 65.6 °C
- Transport Information
- Appearance light yellow low melting crystalline mass
- Safety 7-26-36/37/39-37/39
- Risk Codes 36/37/38-43-42/43
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 3-Buten-2-one,4-phenyl-, (E)- (8CI);(4E)-4-Phenylbut-3-en-2-one;(E)-Benzalacetone;(E)-Benzylideneacetone;(E)-Methyl styryl ketone;Methyl(E)-2-phenylethenyl ketone;Methyl trans-styryl ketone;trans-1-Phenylbut-1-en-3-one;trans-4-Phenyl-3-buten-2-one;
- PSA 17.07000
- LogP 2.28880
Synthetic route

| Conditions | Yield |
|---|---|
| With benzeneseleninic anhydride In dichloromethane for 4h; Ambient temperature; | 100% |
-
-
63511-95-5
(E)-2-methyl-2-styryl-1,3-dioxolane

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With Ru(CH3CN)3(triphos)(OTf)2 (triphos = CH3C(CH2PPh2)3); acetone for 48h; Ambient temperature; | 100% |
| With hydrogenchloride; water In dimethyl sulfoxide at 20℃; Inert atmosphere; |
-
-
75-16-1
methylmagnesium bromide

-
-
80783-99-9, 124931-15-3, 113474-86-5
N-methoxy-N-methylcinnamamide

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; | 100% |
| In tetrahydrofuran; diethyl ether at 0 - 20℃; for 2h; | 98% |
| Stage #1: N-methoxy-N-methylcinnamamide With methyl 4-bromocinnamate; triethylsilyl trifluoromethyl sulfonate; triethylphosphine In toluene at 110℃; for 5h; Inert atmosphere; Stage #2: methylmagnesium bromide In tetrahydrofuran; toluene at -78℃; Inert atmosphere; Stage #3: With tetrabutyl ammonium fluoride In tetrahydrofuran; toluene for 0.5h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 20℃; for 15h; | 100% |
| With potassium carbonate In water at 20℃; for 18h; | 100% |
| With potassium carbonate In water at 20℃; for 18h; other aldehyde; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 100℃; for 18h; Heck reaction; | 100% |
| With tetrabutyl-ammonium chloride; sodium hydrogencarbonate; palladium diacetate In N,N-dimethyl-formamide at 25℃; for 48h; | 98% |
| With triphenylphosphine on reverse phase silica; triethylamine; Pd(OAc)2 on reverse phase silica In methanol; water Addition; Heck reaction; | 97% |
-
-
72047-50-8
2-methyl-2-trans-β-styryl-1,3-dithiane

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; tantalum pentachloride; sodium iodide In water; ethyl acetate at 20℃; for 0.5h; | 100% |
| With [bis(acetoxy)iodo]benzene In water; acetone at 20℃; for 0.0333333h; Ring cleavage; | 90% |
| With o-iodoxybenzoic acid monohydrate In dimethyl sulfoxide at 22 - 32℃; for 16h; | 74% |
| With Dess-Martin periodane In dichloromethane; water; acetonitrile at 20℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 7h; | 99% |
| With sodium hydroxide In water at 20℃; for 6h; Claisen-Schmidt Condensation; | 99% |
| With propylamine; Bi(S(CH2C6H4)2)(H2O)(1+)*CF3(CF2)6CF2SO3(1-)=[Bi(H2O)(S(CH2C6H4)2)](C8F17SO3) In water at 0 - 20℃; for 3h; Claisen-Schmidt condensation; optical yield given as %de; diastereoselective reaction; | 96% |
-
-
36004-04-3
ethyl (E)-1-phenylbut-1-en-3-ol

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; tris(2-methylphenyl)bismuth dichloride In toluene at 20℃; for 0.5h; | 99% |
| With hydrogenchloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium nitrite In dichloromethane; water at 20℃; under 760.051 Torr; for 16h; in air; | 99% |
| With sodium hydroxide; dipotassium peroxodisulfate; nickel(II) sulphate In dichloromethane; water for 24h; Ambient temperature; | 96% |
-
-
73922-81-3, 81555-86-4, 104597-03-7, 5876-76-6
4-phenyl-but-3-yn-2-ol

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With [Rh(±)-2,2’-bis(diphenylphosphino)-1,1’-binaphthyl]BF4 In dichloromethane at 80℃; for 1h; | 99% |
| [Rh(±)-2,2’-bis(diphenylphosphino)-1,1’-binaphthyl]BF4 In 1,2-dichloro-ethane at 80℃; for 1h; | 99% |
| Multi-step reaction with 2 steps 1: manganese(IV) oxide / dichloromethane / 2 h / 20 °C / Darkness; Schlenk technique 2: triphenylphosphine; benzoic acid; water / tetrahydrofuran / 24 h / 65 °C View Scheme |
-
-
51608-60-7
N-(benzylidene)-p-methylbenzenesulfonamide

-
-
1439-36-7
1-triphenylphosphoranylidene-2-propanone

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 100℃; for 12h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| cuprate induced isomerization; | 98% |
| With bis(benzonitrile)palladium(II) dichloride In dichloromethane at 20℃; for 0.333333h; | 97% |
| Beim Erhitzen erfolgt Umwandlung; | |
| cadmium(II) sulphide In dichloromethane Product distribution; Irradiation; |
-
-
100-52-7
benzaldehyde

-
-
1439-36-7
1-triphenylphosphoranylidene-2-propanone

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| In toluene Wittig Olefination; Reflux; | 98% |
| In tetrahydrofuran at 20℃; Wittig reaction; Inert atmosphere; Reflux; optical yield given as %de; | 88% |
| In water at 20℃; for 1h; Wittig reaction; | 85% |
| In tetrahydrofuran at -78 - 20℃; Wittig Olefination; Inert atmosphere; stereoselective reaction; | n/a |

| Conditions | Yield |
|---|---|
| With indium; acetic acid; sodium sulfite In methanol at 20℃; for 0.333333h; | 97.1% |
| With sodium sulfide; Aliquat 336 In water; benzene for 1h; Ambient temperature; | 84% |
| With N,N-dimethyl-formamide In hydrogen fluoride at 160℃; for 1h; | 74% |
-
-
32398-66-6
1-phenylbut-2-yn-1-ol

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]-[bis(trifluoromethanesulfonyl)-imide]gold(I) In water; isopropyl alcohol at 40℃; for 2.5h; Meyer-Schuster Rearrangement; | 97% |
| With indium(III) chloride In water at 160℃; for 0.166667h; Meyer-Schuster rearrangement; Microwave irradiation; stereoselective reaction; | 89% |
| With methanol; (triphenylphosphine)gold(I) chloride; oxygen; silver(I) triflimide In dichloromethane at 25℃; for 24h; | 72% |
-
-
591-50-4
iodobenzene

-
-
78-94-4
methyl vinyl ketone

-
A
-
2550-26-7
4-Phenyl-2-butanone

-
B
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 120℃; for 16h; Catalytic behavior; Reagent/catalyst; Temperature; Heck Reaction; Overall yield = 100 %; | A 3% B 97% |
| palladated Kaiser oxime resin In water at 120℃; for 20h; Heck reaction; | A 10% B 82% |
| With 1-ethyl-3-methyl-1H-imidazol-3-ium methyl phosphonate at 150℃; for 16h; Catalytic behavior; Reagent/catalyst; Temperature; Heck Reaction; Overall yield = 100 %; | A 80% B 20% |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 50℃; for 36h; Wittig reaction; optical yield given as %de; | 96% |
| With 2-propenamide; triphenylphosphine In propan-1-ol at 20 - 90℃; for 5h; Wittig reaction; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 93% |
| With cerium(III) triiodide In tetrahydrofuran for 1h; Ambient temperature; | 80% |

| Conditions | Yield |
|---|---|
| With silver(I) tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; C19H13I2N3O2Ru; hydrogen In water at 80℃; under 3750.38 Torr; for 4h; Autoclave; Schlenk technique; chemoselective reaction; | 96% |
| With formic acid; gold nanoparticles on rutile titania; triethylamine In acetone at 60℃; for 1h; stereoselective reaction; | 89% |
| With TPPMS (meta-monosulfonated triphenylphosphane, Na salt); water for 3h; |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tetrabutyl-ammonium chloride; sodium hydrogencarbonate In N,N-dimethyl-formamide at 100℃; for 4h; Heck Reaction; Microwave irradiation; | 96% |
| With triethylamine In N,N-dimethyl-formamide at 100℃; for 18h; Heck reaction; | 88% |

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; trifluoroacetic acid In dimethyl sulfoxide at 80℃; for 6h; Sealed tube; | 96% |
| With iron(III) chloride; 1,10-Phenanthroline; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 48h; Inert atmosphere; Schlenk technique; | 69% |
| Multi-step reaction with 2 steps 1: 2,6-dimethylpyridine / tetrahydrofuran / 1 h / 0 °C 2: eosin y; potassium acetate; oxygen / ethanol / 4 °C / Irradiation View Scheme | |
| With iodine; N,N-dimethyl-formamide; copper(II) oxide at 100℃; for 24h; Inert atmosphere; | 111 mg |
-
-
5381-93-1, 86734-67-0, 86734-69-2, 127707-68-0
1-hydroxy-1-phenyl-3-butanone

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 25℃; for 24h; | 95% |
| With cerium(III) chloride; sodium iodide In acetonitrile for 10h; Dehydration; Heating; | 89% |
| In diethyl ether for 3h; | |
| With hydrogenchloride In water at 60℃; for 1h; | 54.8 mg |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; for 1h; | 95% |
-
-
72047-50-8
2-methyl-2-trans-β-styryl-1,3-dithiane

-
A
-
109-80-8
1.3-propanedithiol

-
B
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; water; methylene green In acetonitrile Irradiation; | A n/a B 94% |
-
-
909413-25-8
6-(tert-butyl)-12-phenyl-5,6,7,12-tetrahydrodibenzo[c,f][1,5]azastibocine

-
-
78-94-4
methyl vinyl ketone

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With palladium dichloride In N,N-dimethyl acetamide at 80℃; for 6h; Catalytic behavior; Reagent/catalyst; Solvent; Heck Reaction; | 94% |
-
-
43100-94-3
(3-Oxo-1-phenyl-butyl)-triphenyl-phosphonium; perchlorate

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; chloroform Ambient temperature; | 93% |
-
-
36004-04-3
ethyl (E)-1-phenylbut-1-en-3-ol

-
A
-
4426-63-5
1-Phenyl-1,2-epoxy-butanol-(3)

-
B
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With oxo(salen)chromium(V)+ PF6- In dichloromethane at 20℃; for 1h; Product distribution; Further Variations:; Reagents; Oxidation; epoxidation; | A n/a B 93% |
| With CrIII(salen)(TfO) In dichloromethane at 20℃; for 1h; Product distribution; Further Variations:; Reagents; Oxidation; epoxidation; |
-
-
100-52-7
benzaldehyde

-
-
67-64-1
acetone

-
A
-
5381-93-1, 86734-67-0, 86734-69-2, 127707-68-0
1-hydroxy-1-phenyl-3-butanone

-
B
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With pyrrolidine In water for 0.0833333h; | A 93% B 6% |
| [Choline][Pro] In water at 20℃; for 12h; | A 80.4% B 8% |
| With sodium hydroxide In water for 7h; | A 19% B 77% |

-
-
100-52-7
benzaldehyde

-
-
67-63-0
isopropyl alcohol

-
A
-
1896-62-4
(E)-benzalacetone

-
B
-
100-51-6
benzyl alcohol

| Conditions | Yield |
|---|---|
| at 225℃; for 24h; | A n/a B 92% |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol at 225℃; for 24h; | A 2% B 92% |
| With isopropyl alcohol at 225℃; for 24h; | A n/a B 92% |
-
-
6310-44-7, 32147-15-2, 55025-54-2
erythro-3.4-Dibrom-4-phenyl-butan-2-on

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| With indium; iron(III) chloride hexahydrate In methanol at 20℃; for 1h; chemoselective reaction; | 92% |
| With bismuth(III) chloride; gallium In tetrahydrofuran at 20℃; for 0.5h; stereoselective reaction; | 91% |
| With iron In methanol for 0.416667h; Dehalogenation; Heating; | 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 20h; Wittig olefination; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride; sodium formate In water; 1,2-dichloro-benzene at 108.9℃; for 0.166667h; | 100% |
| With dicobalt octacarbonyl; water In 1,2-dimethoxyethane for 2h; Heating; | 100% |
| With 1-ethyl-3-methyl-1H-imidazol-3-ium methyl phosphonate; hydrogen at 30℃; under 15001.5 Torr; for 16h; Catalytic behavior; Reagent/catalyst; Temperature; Schlenk technique; | 100% |
-
-
1896-62-4
(E)-benzalacetone

-
-
36004-04-3
ethyl (E)-1-phenylbut-1-en-3-ol

| Conditions | Yield |
|---|---|
| With Zn(BH4)2(Ph3P)2 at 60℃; for 0.16h; Reduction; | 100% |
| With sodium tetrahydroborate In methanol for 2h; Cooling with ice; | 100% |
| With diisobutylaluminium hydride In hexane; toluene at -78℃; for 3h; or LiAlH(i-Bu)2(n-Bu); | 99% |
-
-
1896-62-4
(E)-benzalacetone

-
-
21613-44-5
(E)-4-phenyl-3-buten-2-one oxime

| Conditions | Yield |
|---|---|
| With pyridine; hydroxylamine hydrochloride In ethanol at 60℃; for 1h; | 100% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 80℃; for 4h; Inert atmosphere; chemoselective reaction; | 59% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water for 5h; Heating; | 52% |

| Conditions | Yield |
|---|---|
| With tributyl-amine; tetra-(n-butyl)ammonium iodide; trifluoroacetic acid; bis(triphenylphosphine) palladium (Il) acetate In N,N-dimethyl-formamide at 70℃; | 100% |
| With triethylamine; palladium diacetate In carbon dioxide at 80℃; under 75006 Torr; for 60h; Arylation; Hydroarylation; | 88% |
-
-
6651-36-1
1-(Trimethylsilyloxy)cyclohexene

-
-
1896-62-4
(E)-benzalacetone

-
-
221363-48-0
2-(3'-oxo-1'-phenylbutyl)-1-cyclohexanone

| Conditions | Yield |
|---|---|
| tin(IV) chloride; zinc(II) chloride In dichloromethane at -78℃; for 3h; | 100% |
| chloro-trimethyl-silane; tin(ll) chloride In dichloromethane at -78℃; for 13h; | 93% |
| With lithium perchlorate In nitromethane for 16h; Ambient temperature; | 85% |
| With hydrogenchloride; bismuth(III) chloride 1) CH2Cl2, rt, 6 h, 2) MeOH; Yield given. Multistep reaction; |
-
-
1896-62-4
(E)-benzalacetone

-
-
62413-47-2
(2R,3E)-4-phenyl-3-buten-2-ol

| Conditions | Yield |
|---|---|
| With (S,S)-RuCl2(2,2'-bis(di-3,5-xylylphosphino)-1,1'-binaphthyl)(1,1-dianisyl-2-isopropyl-1,2-ethylenediamine); hydrogen; potassium carbonate In isopropyl alcohol at 28 - 30℃; under 60800 Torr; for 43h; | 100% |
| Stage #1: (E)-benzalacetone With C51H80N4O4; scandium tris(trifluoromethanesulfonate) In tetrahydrofuran at 35℃; for 0.5h; Stage #2: With potassium borohydride In tetrahydrofuran; water at 0℃; for 1.5h; enantioselective reaction; | 99% |
| With benzo[1,3,2]dioxaborole; Oxazaborolidine 2 In toluene at -78℃; for 15h; | 95% |
-
-
80522-42-5
triisopropylsilyl trifluoromethanesulfonate

-
-
1896-62-4
(E)-benzalacetone

-
-
163811-53-8
(E)-triisopropyl((4-phenylbuta-1,3-dien-2-yl)oxy)silane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 8h; Inert atmosphere; | 100% |
| With triethylamine In tetrahydrofuran at 0℃; for 2.5h; Inert atmosphere; Schlenk technique; | 88% |
| With triethylamine In benzene at 20℃; for 2h; | 62% |
| In dichloromethane |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 50℃; | 100% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
1896-62-4
(E)-benzalacetone

-
-
18931-64-1
(E)-1,1,1-trifluoro-6-phenylhex-5-ene-2,4-dione

| Conditions | Yield |
|---|---|
| With sodium hydride at 40℃; for 3h; | 100% |
| With lithium hydride In benzene for 5h; Condensation; | 65% |
-
-
100-63-0
phenylhydrazine

-
-
1896-62-4
(E)-benzalacetone

-
-
358723-67-8
(E)-1-phenyl-2-((E)-4-phenylbut-3-en-2-ylidene)hydrazine

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0833333h; | 100% |
| With acetic acid In methanol at 20℃; Inert atmosphere; | 85% |
| With acetic acid In methanol for 0.75h; Heating; | |
| With acetic acid In methanol at 20℃; |
-
-
98-86-2
acetophenone

-
-
1896-62-4
(E)-benzalacetone

-
A
-
1445-91-6
(S)-1-phenylethanol

-
B
-
1517-69-7
(R)-1-phenylethanol

| Conditions | Yield |
|---|---|
| With Triethoxysilane; (S,S,S,S)-N,N'-di(α-phenylethyl)cyclohexane-1,2-diamine; diethylzinc In toluene at 20℃; for 18h; Title compound not separated from byproducts.; | A n/a B 100% |
| With polymethylhydrosiloxane; (S,S,S,S)-N,N'-di(α-phenylethyl)cyclohexane-1,2-diamine; diethylzinc In toluene at 20℃; for 24h; Title compound not separated from byproducts.; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With polymer-micelle incarcerated Sc(OTf)3 In acetonitrile at 20℃; for 6h; Michael reaction; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-indene-1,3(2H)-dione; 4-chlorobenzaldehyde With L-proline In methanol at 20℃; for 0.5h; organocatalytic Knoevenagel condensation; Stage #2: (E)-benzalacetone In methanol at 25℃; for 96h; Diels-Alder/epimerization reaction; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: 1H-indene-1,3(2H)-dione; 1-naphthaldehyde With L-proline In methanol at 20℃; for 0.5h; organocatalytic Knoevenagel condensation; Stage #2: (E)-benzalacetone In methanol at 25℃; for 96h; Diels-Alder/epimerization reaction; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: 1H-indene-1,3(2H)-dione; 4-hydroxy-benzaldehyde With L-proline In methanol at 20℃; for 0.5h; organocatalytic Knoevenagel condensation; Stage #2: (E)-benzalacetone In methanol at 25℃; for 96h; Diels-Alder/epimerization reaction; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: 1H-indene-1,3(2H)-dione; 4-cyanobenzaldehyde With L-proline In methanol at 20℃; for 0.5h; organocatalytic Knoevenagel condensation; Stage #2: (E)-benzalacetone In methanol at 25℃; for 96h; Diels-Alder/epimerization reaction; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: 1H-indene-1,3(2H)-dione; methyl 4-formylbenzoate With L-proline In methanol at 20℃; for 0.5h; organocatalytic Knoevenagel condensation; Stage #2: (E)-benzalacetone In methanol at 25℃; for 96h; Diels-Alder/epimerization reaction; | 100% |

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
1896-62-4
(E)-benzalacetone

-
-
76217-07-7
4-(4-methoxyphenyl)-4-phenylbutan-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide; [{Rh(C2H4)2Cl}2]; (-)-borneol biphenol phosphite In 1,4-dioxane; water at 80℃; for 15h; | 100% |
| Rh(dpm)(CO)2; 1,3-bis(2-diphenylphosphinomethylphenyl)benzene In cyclohexane; water at 60℃; for 16h; | 98% |
| With potassium phosphate; ferrocenyl-containing palladacycle In toluene at 20℃; | 93% |
| With 4,4’‐bis(trimethylammoniummethyl)‐2,2’‐bipyridine; tetrafluoroboric acid; diamminedichloropalladium(II) In water at 80℃; for 24h; pH=1; | 88% |
-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
1896-62-4
(E)-benzalacetone

-
-
100840-12-8
(E)-3-methyl-1-phenylhexa-1,5-dien-3-ol

| Conditions | Yield |
|---|---|
| With indium iodide In tetrahydrofuran at 40℃; for 24h; | 100% |
| With ethanol; diethylzinc In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 93% |
| With tricyclohexylphosphine; bis(1,5-cyclooctadiene)nickel (0) In tetrahydrofuran at 65℃; for 12h; |

-
-
1896-62-4
(E)-benzalacetone

| Conditions | Yield |
|---|---|
| Stage #1: ethyl bromozincacetate;THF; (E)-benzalacetone In tetrahydrofuran at 0 - 25℃; for 3h; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 48h; Molecular sieve; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 24h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| (HN(iPr2PC2H4)2)IrH3 In isopropyl alcohol at 25℃; for 1 - 2h; Conversion of starting material; | 99% |
| With potassium tert-butylate; IrH2Cl[(iPr2PC2H4)2NH] In isopropyl alcohol at 25℃; for 1 - 2h; Conversion of starting material; | 99% |
| With tetrakis[3,5-bis(trifluoromethyl)phenyl]boric acid bis(diethyl ether) complex; (bis[(2-dicyclohexylphosphino)ethyl]amine)cobalt(II)(CH2SiMe3); isopropyl alcohol In tetrahydrofuran at 25℃; for 24h; Inert atmosphere; Glovebox; Schlenk technique; Sealed tube; | 98% |
(E)-4-Phenyl-3-buten-2-one Chemical Properties
IUPAC Name: (E)-4-Phenylbut-3-en-2-one
Molecular formula: C10H10O
Molecular Weight: 146.19 g/mol
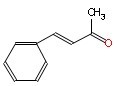
The Molecular Structure of trans-Benzalacetone (CAS NO.1896-62-4):
EINECS: 204-555-1
Melting Point: 39-42 °C(lit.)
Boiling Point: 260.8 °C at 760 mmHg
Flash Point: 65.6 °C
Index of Refraction: 1.563
Molar Refractivity: 46.8 cm3
Molar Volume: 144 cm3
Polarizability: 18.55×10 -24 cm3
Surface Tension: 36.9 dyne/cm
Density: 1.014 g/cm3
Enthalpy of Vaporization: 49.85 kJ/mol
Vapour Pressure: 0.012 mmHg at 25 °C
Water Solubility: practically insoluble
XLogP3: 2.1
H-Bond Acceptor: 1
Rotatable Bond Count: 2
Tautomer Count: 2
Exact Mass: 146.073165
MonoIsotopic Mass: 146.073165
Topological Polar Surface Area: 17.1
Canonical SMILES: CC(=O)C=CC1=CC=CC=C1
Isomeric SMILES: CC(=O)/C=C/C1=CC=CC=C1
InChI: InChI=1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+
InChIKey: BWHOZHOGCMHOBV-BQYQJAHWSA-N
Product Categories: C10; Carbonyl Compounds; Ketones
(E)-4-Phenyl-3-buten-2-one Toxicity Data With Reference
| 1. | mma-sat 300 µg/plate | FCTOD7 Food and Chemical Toxicology, 20 (1982),427. |
(E)-4-Phenyl-3-buten-2-one Safety Profile
Hazard Codes:  Xn,
Xn,  Xi
Xi
Risk Statements: 36/37/38-43-42/43
R36/37/38:Irritating to eyes, respiratory system and skin.
R43:May cause sensitization by skin contact.
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements: 7-26-36/37/39-37/39
S7:Keep container tightly closed.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
RTECS: EN0330050
F: 8
Mutation data reported. A flammable liquid. When heated to decomposition it emits acrid smoke and irritating vapors.
(E)-4-Phenyl-3-buten-2-one Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
(E)-4-Phenyl-3-buten-2-one Specification
trans-Benzalacetone (CAS NO.1896-62-4) is also named as 4-07-00-01003 (Beilstein Handbook Reference) ; AI3-52291 ; BRN 0742047 ; Methyl trans-styryl ketone ; TPBO ; trans-4-Phenyl-3-butene-2-one ; trans-Benzalacetone ; trans-Benzylidenacetone ; trans-Benzylideneacetone . trans-Benzalacetone (CAS NO.1896-62-4) is light yellow low melting crystalline mass. It is flammable. It will produce stimulate smoke when buring. So the storage environment should be ventilate, low-temperature and dry.
Related Products
- (E)-4-Phenyl-3-buten-2-one
- 18967-31-2
- 18967-35-6
- 18967-41-4
- 18967-44-7
- 189680-06-6
- 18968-05-3
- 189681-04-7
- 18968-14-4
- 189684-53-5
- 189684-54-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View