-
Name
2'-Fluoroacetophenone
- EINECS 207-156-0
- CAS No. 445-27-2
- Article Data69
- CAS DataBase
- Density 1.103 g/cm3
- Solubility
- Melting Point 26-27 °C
- Formula C8H7FO
- Boiling Point 180.8 °C at 760 mmHg
- Molecular Weight 138.141
- Flash Point 67.5 °C
- Transport Information UN 2810
- Appearance Clear colorless to light yellow or light green
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetophenone,2'-fluoro- (6Cl,7Cl,8Cl);1-(2-Fluorophenyl)ethanone;2-Fluorophenyl methylketone;NSC 88297;o-Fluoroacetophenone;
- PSA 17.07000
- LogP 2.02830
Synthetic route

| Conditions | Yield |
|---|---|
| With [(2-(benzoimidazol-2-yl)-6-(3,5-dimethylpyrazol-1-yl)pyridine)RuCl2(PPh3)]; potassium tert-butylate; acetone In methanol at 56℃; under 750.075 Torr; for 0.5h; Catalytic behavior; Oppenauer Oxidation; Inert atmosphere; | 97% |
| With Langlois reagent In acetonitrile at 25℃; for 12h; Irradiation; Sealed tube; | 89% |
| With calcomenite; potassium hydroxide In toluene for 28h; Reflux; | 87% |

| Conditions | Yield |
|---|---|
| With iron(III) trifluoromethanesulfonate; C65H77N5O4S2; oxygen In 1,2-dichloro-ethane at 75℃; under 760.051 Torr; for 24h; Green chemistry; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With C22H20AuN3O2P(1+)*CF3O3S(1-); water; silver trifluoromethanesulfonate; acetic acid at 100℃; for 10h; | 93% |
| With silver trifluoromethanesulfonate In water; acetic acid at 110℃; for 6h; Schlenk technique; | 87% |
| With p-toluenesulfonic acid monohydrate; acetic acid In dichloromethane at 80℃; for 8h; Sealed tube; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: methyllithium With copper(l) cyanide In diethyl ether at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: o-fluoro-benzoic acid In diethyl ether at 0 - 20℃; for 15h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; sodium hydrogencarbonate In water at 100℃; for 5h; Autoclave; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl malonate With ethanol; magnesium In tetrachloromethane; tert-butyl methyl ether for 3h; Reflux; Large scale; Stage #2: 2-Fluorobenzoyl chloride In tert-butyl methyl ether for 0.25h; Reflux; Large scale; Stage #3: With sulfuric acid; water; acetic acid In tert-butyl methyl ether for 4h; Reagent/catalyst; Reflux; Large scale; | 83% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; silver nitrate; N-fluorobis(benzenesulfon)imide; methyl carbamate In dichloromethane at 95℃; for 12h; Catalytic behavior; Solvent; Sealed tube; | 82% |
| Multi-step reaction with 3 steps 1: sodium acetate / ethanol; water / 2 h / Reflux 2: tris(dibenzylideneacetone)dipalladium(0) chloroform complex; N-fluorobis(benzenesulfon)imide; potassium nitrate / nitromethane / 24 h / 25 °C 3: hydrogenchloride; water / diethyl ether / 30 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; diisopropylamine; 1-butyl-3-methylimidazolium Tetrafluoroborate; palladium diacetate In dimethyl sulfoxide at 115℃; for 36h; Heck arylation; | 81% |

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; water; palladium diacetate at 100℃; under 760.051 Torr; for 6h; | 81% |
-
-
401514-42-9
1-(2,2-dibromovinyl)-2-fluorobenzene

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With water; zinc at 275℃; for 4h; | 79% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); N-ethyl-N,N-diisopropylamine; lithium chloride In N,N-dimethyl-formamide at 100℃; for 8.5h; | 74% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water; palladium diacetate; 2-(3,5-dimethyl-1H-pyrazol-1-yl)pyridine at 60℃; for 5h; regioselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; C21H19N5Pd(2+)*2BF4(1-) In decane; acetonitrile at 45℃; for 30h; Wacker Oxidation; | 70% |
| With manganese(II) bromide; water; lithium perchlorate; copper dichloride In acetonitrile at 60℃; for 8h; Wacker-Tsuji Olefin Oxidation; Sealed tube; Inert atmosphere; Electrochemical reaction; regioselective reaction; | 46% |
| With oxygen; potassium carbonate; isopropyl alcohol at 150℃; under 3000.3 Torr; for 6h; regioselective reaction; | 82.3 %Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: 2'-fluoroacetophenone oxime With hexachlorodisilane; silica gel In toluene at 110℃; for 8h; Stage #2: With water In toluene for 0.5h; | 67% |
| With ruthenium trichloride; toluene-4-sulfonic acid In N,N-dimethyl acetamide; water at 120℃; under 760.051 Torr; for 8h; Inert atmosphere; Green chemistry; | 61% |
-
-
1547526-82-8
2-(2-fluorophenyl)propane-1,2-diol

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With oxygen; sodium carbonate In water at 20℃; for 5h; Irradiation; Green chemistry; | 51% |

| Conditions | Yield |
|---|---|
| With oxygen at 150℃; under 760.051 Torr; for 24h; Autoclave; | 36% |

| Conditions | Yield |
|---|---|
| With dimethylcadmium; benzene |
-
-
128254-28-4
1-(2-Fluoro-4-trimethylsilanyl-phenyl)-ethanone

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With potassium fluoride In N,N-dimethyl-formamide at -40℃; for 3h; Yield given; |

| Conditions | Yield |
|---|---|
| With copper 1) THF, 25 deg C, 10 min, 2a) 25 deg C, 30 min; Yield given. Multistep reaction; | |
| With CuI*P(Et)3; naphthalen-1-yl-lithium 1.) DME, 25 deg C, 10 min, 2.) 25 deg C, 30 min; Yield given. Multistep reaction; |
-
-
98-86-2
acetophenone

-
A
-
445-27-2
2'-Fluoroacetophenone

-
B
-
403-42-9
1-(4-fluorophenyl)ethanone

-
C
-
455-36-7
1-(3-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With cobalt (III) fluoride In chloroform for 18h; Product distribution; Heating; |

-
-
128254-27-3
2-Fluoro-1,4-bis-trimethylsilanyl-benzene

-
-
75-36-5
acetyl chloride

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With aluminium trichloride; water; fluoride Multistep reaction; |
-
-
7789-21-1
fluorosulphonic acid

-
-
98-86-2
acetophenone

-
A
-
445-27-2
2'-Fluoroacetophenone

-
B
-
18355-80-1
2,3-difluoroacetophenone

| Conditions | Yield |
|---|---|
| at 110℃; |


| Conditions | Yield |
|---|---|
| Erhitzen des von Aether befreiten Reaktionsgemisches auf dem Dampfbad und anschliessendes Behandeln mit wss. Essigsaeure; |
-
-
98-86-2
acetophenone

-
A
-
445-27-2
2'-Fluoroacetophenone

-
B
-
69291-63-0
1-(3,3,6,6-tetrafluoro-1,4-cyclohexyldienyl)-1-ethanone

-
C
-
1979-36-8
2',5'-difluoroacetophenone

-
D
-
455-36-7
1-(3-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With hydrogen fluoride In diethyl ether; acetone at 5℃; for 18h; Electrochemical reaction; |

-
-
839721-38-9
1-(1-butoxy-vinyl)-2-fluoro-benzene

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.5h; | |
| With hydrogenchloride; water In isopropyl alcohol for 0.5h; | |
| With hydrogenchloride; water In ethylene glycol Inert atmosphere; | |
| With hydrogenchloride; water In diethyl ether at 20℃; for 1h; Inert atmosphere; | |
| With hydrogenchloride In acetone at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1,3-bis(diphenylphosphino)propane; Pd(OAc)2; Et3N / 1-butyl-3-methylimidazolium tetrafluoroborate / 36 h / 115 °C 2: aq. HCl / 0.5 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether 2: pyridinium chlorochromate / CH2Cl2 View Scheme |
-
-
128254-27-3
2-Fluoro-1,4-bis-trimethylsilanyl-benzene

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 2: KF / dimethylformamide / 3 h / -40 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: methylmagnesium bromide; 2-Fluorobenzaldehyde In tetrahydrofuran Stage #2: With pyridinium chlorochromate In dichloromethane |

| Conditions | Yield |
|---|---|
| Stage #1: 2'-Fluoroacetophenone With C107H90Cl2N10P4Ru2(2+)*2Cl(1-) In isopropyl alcohol at 82℃; for 0.166667h; Inert atmosphere; Stage #2: With potassium isopropoxide In isopropyl alcohol for 2h; Catalytic behavior; Inert atmosphere; | 100% |
| With C40H37ClN2PRuS(1+)*C24H20B(1-); isopropyl alcohol; potassium hydroxide at 82℃; for 2h; Catalytic behavior; | 100% |
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; sodium formate; ephedrine hydrochloride at 20℃; | 99% |
-
-
445-27-2
2'-Fluoroacetophenone

-
A
-
171032-87-4
(1S)-1-(2-fluorophenyl)ethanol

-
B
-
162427-79-4
(R)-1-(2-fluorophenyl)ethanol

| Conditions | Yield |
|---|---|
| With RuBr2[(S,S)-2,4-bis(diphenylphosphino)pentane](2-picolylamine); potassium tert-butylate; hydrogen In ethanol at 40℃; under 7600.51 Torr; for 19h; Inert atmosphere; Autoclave; | A 100% B n/a |
| With dimethylsulfide borane complex; (S)-tetrahydro-1-butyl-3,3-diphenyl-1H,3H-pyrrolo{2,1-c}{1,3,2}oxazaborole In tetrahydrofuran for 0.166667h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With Cp*RhCl<(R,R)-Tscydn>; potassium tert-butylate In isopropyl alcohol at 30℃; for 12h; Yield given; Yields of byproduct given. Title compound not separated from byproducts; |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
171032-87-4
(1S)-1-(2-fluorophenyl)ethanol

| Conditions | Yield |
|---|---|
| With [((R,R)-diphenylethylenediamine)((R)-Binap)dichlororuthenium(II)]; potassium tert-butylate; hydrogen In isopropyl alcohol at 26 - 30℃; under 3040 Torr; for 3h; | 100% |
| With tris(bis(trimethylsilyl)amido)lanthanum(III); 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane; ((2S,2'S)-((2-hydroxy-5-methyl-1,3-phenylene)bis(methylene))bis(pyrrolidine-1,2-diyl))bis(diphenylmethanol) In tetrahydrofuran at 0℃; for 8h; enantioselective reaction; | 99% |
| In phosphate buffer for 48h; Microbiological reaction; | 98% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
162427-79-4
(R)-1-(2-fluorophenyl)ethanol

| Conditions | Yield |
|---|---|
| With (S,S)-RuCl2(2,2'-bis(di-3,5-xylylphosphino)-1,1'-binaphthyl)(1,1-dianisyl-2-isopropyl-1,2-ethylenediamine); potassium tert-butylate; hydrogen In isopropyl alcohol at 26 - 30℃; under 6080 Torr; for 13h; | 100% |
| With water; sodium formate; sodium dodecyl-sulfate at 39.84℃; for 96h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 95% |
| With bromopentacarbonylmanganese(I); hydrogen; C45H43FeNP2; potassium hydroxide In methanol; toluene at 20℃; for 36h; enantioselective reaction; | 93% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
2627-86-3
(S)-1-phenyl-ethylamine

-
-
444643-06-5
[1-(3-Fluoro-phenyl)-eth-(E)-ylidene]-((S)-1-phenyl-ethyl)-amine

| Conditions | Yield |
|---|---|
| zinc(II) chloride In toluene for 15h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water at 95℃; | 100% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 25℃; for 4h; | 94.7% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water Heating; |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 12h; Reflux; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In toluene at -43 - 20℃; for 6.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 1h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| N,N,N',N'-tetramethylguanidine at 25℃; for 15h; | 99% |
| With C42H50Mg2N4 In benzene-d6 at 25℃; for 0.8h; Glovebox; Inert atmosphere; | 99% |
| Fe(Cp)2PF6 at 20℃; for 0.166667h; | 94% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
75-33-2
2-propanethiol

-
-
918811-12-8
1-(2-(isopropylthio)phenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; | 99% |
-
-
120-57-0
piperonal

-
-
445-27-2
2'-Fluoroacetophenone

-
-
1352346-99-6
(E)-3-(benzo[d][1,3]dioxol-5-yl)-1-(2-fluorophenyl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2'-Fluoroacetophenone With C38H26LiO5P In hexane; water; toluene at -78℃; for 0.166667h; Inert atmosphere; Stage #2: trimethylsilyl cyanide In hexane; water; toluene at -78℃; for 4h; Inert atmosphere; enantioselective reaction; | 99% |

-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; C51H41F4NO6P2; mesitylcopper(I) at 30℃; for 40h; diastereoselective reaction; | 99% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
130581-22-5
3-(4-methoxyphenyl)-1-(2-fluorophenyl)-2-propen-1-one

| Conditions | Yield |
|---|---|
| With methanol; sodium In methanol at 20℃; Aldol Condensation; | 98.4% |
-
-
129217-85-2
trimethyl (3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)prop-1-yn-1-yl)silane

-
-
445-27-2
2'-Fluoroacetophenone

-
-
1312791-19-7
(R)-2-(2-fluorophenyl)-5-(trimethylsilyl)pent-4-yn-2-ol

| Conditions | Yield |
|---|---|
| With copper (II) isobutyrate; (R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl; lithium tert-butoxide In tetrahydrofuran at -62℃; for 16h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 20℃; for 2h; | 97% |
| With bromine In acetic acid at 20℃; for 2h; | 97% |
| With copper(I) bromide In ethyl acetate at 80℃; | 95% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
79110-05-7
1-(2-fluoro-5-nitrophenyl)ethanone

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at -42℃; for 0.5h; | 97% |
| Stage #1: 2'-Fluoroacetophenone With sulfuric acid; nitric acid at -40℃; for 1.08333h; Stage #2: With water Cooling with ice; | 97% |
| With sulfuric acid; nitric acid In water at -10 - 5℃; for 0.5h; | 93.7% |

| Conditions | Yield |
|---|---|
| With iodine at 100℃; | 97% |
| With iodine at 100℃; for 8h; | 66% |
| With iodine; dimethyl sulfoxide at 80℃; for 12h; Schlenk technique; | 52% |
-
-
445-27-2
2'-Fluoroacetophenone

| Conditions | Yield |
|---|---|
| Stage #1: 2'-Fluoroacetophenone; N-carbethoxy-4-tropinone With Nonafluorobutanesulfonyl fluoride; tert-butylimino-tri(pyrrolidino)phosphorane; lithium chloride In N,N-dimethyl-formamide at 10 - 25℃; Stage #2: With copper(l) iodide; diisopropylamine; triphenylphosphine; palladium diacetate In N,N-dimethyl-formamide at 60℃; for 5.5h; Sonogashira coupling; | 97% |

-
-
445-27-2
2'-Fluoroacetophenone

-
-
506-87-6
ammonium carbonate

-
-
7248-25-1
(RS)-5-(2-fluoro-phenyl)-5-methyl-imidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In ethanol; water at 60℃; for 5.5h; | 97% |
| With ammonium hydroxide In ethanol; water at 60℃; for 5.5h; | 97% |
| In ethanol at 50℃; for 24h; Bucherer-Bergs Reaction; |

-
-
445-27-2
2'-Fluoroacetophenone

-
-
134469-07-1
Benzimidazol-2-thiol

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid for 0.5h; Reflux; | 97% |

| Conditions | Yield |
|---|---|
| With C7H13N2O3S(1+)*C10H15O4S(1-) at 25℃; for 0.25h; Mannich Aminomethylation; | 97% |

-
-
445-27-2
2'-Fluoroacetophenone

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
31310-22-2
ethyl 3-methyl-1-benzothiophene-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 75℃; for 16h; Inert atmosphere; | 97% |
-
-
445-27-2
2'-Fluoroacetophenone

-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
1071821-42-5
2-(2-fluorophenyl)pent-4-en-2-ol

| Conditions | Yield |
|---|---|
| With ethanol; diethylzinc In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 96% |
| With indium In water at 30℃; for 24h; Inert atmosphere; | 91% |
-
-
5344-90-1
2-Aminobenzyl alcohol

-
-
445-27-2
2'-Fluoroacetophenone

-
-
2836-41-1
2-(2-fluorophenyl)quinoline

| Conditions | Yield |
|---|---|
| With C22H24ClN3ORu; potassium hydroxide In toluene at 80℃; for 5h; Catalytic behavior; Green chemistry; | 96% |
| With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate In tetrahydrofuran at 20℃; for 12h; | 96% |
| With caesium carbonate In toluene at 120℃; for 10h; Schlenk technique; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| With C7H13N2O3S(1+)*C10H15O4S(1-) at 25℃; for 0.416667h; Mannich Aminomethylation; | 96% |


| Conditions | Yield |
|---|---|
| With C7H13N2O3S(1+)*C10H15O4S(1-) at 25℃; for 0.416667h; Mannich Aminomethylation; | 96% |

2'-Fluoroacetophenone Specification
The 2'-Fluoroacetophenone with CAS registry number of 445-27-2 is also known as 2-Fluorophenyl methylketone. The IUPAC name is 1-(2-Fluorophenyl)ethanone. It belongs to product categories of Acetophenone Series; Aromatic Acetophenones & Derivatives (substituted); Fluorobenzene; Ketone; Adehydes, Acetals & Ketones; Fluorine Compounds; C7 to C8; Carbonyl Compounds; Ketones. Its EINECS registry number is 207-156-0. In addition, the formula is C8H7FO and the molecular weight is 138.14. This chemical is a clear colorless to light yellow or light green and should be stored in sealed containers away from oxidizing agents, water, alkali, alcohol and amine.
Physical properties about 2'-Fluoroacetophenone are: (1)ACD/LogP: 1.30; (2)ACD/LogD (pH 5.5): 1.29; (3)ACD/LogD (pH 7.4): 1.29; (4)ACD/BCF (pH 5.5): 5.67; (5)ACD/BCF (pH 7.4): 5.67; (6)ACD/KOC (pH 5.5): 120.58; (7)ACD/KOC (pH 7.4): 120.58; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.491; (11)Molar Refractivity: 36.27 cm3; (12)Molar Volume: 125.1 cm3; (13)Surface Tension: 32.8 dyne/cm; (14)Density: 1.103 g/cm3; (15)Flash Point: 67.5 °C; (16)Enthalpy of Vaporization: 41.71 kJ/mol; (17)Boiling Point: 180.8 °C at 760 mmHg; (18)Vapour Pressure: 0.88 mmHg at 25 °C.
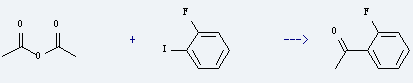
Preparation of 2'-Fluoroacetophenone: it is prepared by reaction of acetic acid anhydride with 1-fluoro-2-iodo-benzene. The reaction needs reagents diisopropylethylamine, Pd2(dba)3, lithium chloride and solvent dimethylformamide at the temperature of 100 °C for 8.5 hours. The yield is about 74%.
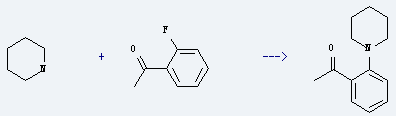
Uses of 2'-Fluoroacetophenone: it is used to produce 1-(2-piperidin-1-yl-phenyl)-ethanone by reaction with piperidine. The reaction occurs with reagent K2CO3 and solvent dimethylsulfoxide for 8 hours. The yield is about 80%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC(=O)C1=CC=CC=C1F
2. InChI: InChI=1S/C8H7FO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,1H3
3. InChIKey: QMATYTFXDIWACW-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View