-
Name
2-Butyl-1H-imidazole-4-carbaldehyde
- EINECS 614-393-7
- CAS No. 68282-49-5
- Article Data16
- CAS DataBase
- Density 1.099 g/cm3
- Solubility
- Melting Point 118-119 oC
- Formula C8H12N2O
- Boiling Point 359.1 °C at 760 mmHg
- Molecular Weight 152.196
- Flash Point 174.8 °C
- Transport Information
- Appearance white solid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1H-Imidazole-4-carboxaldehyde,2-butyl- (9CI);2-Butyl-1H-imidazole-4-carboxaldehyde;2-Butyl-1H-imidazole-5-carboxaldehyde;2-Butyl-3H-imidazole-4-carboxaldehyde;2-Butyl-4-formylimidazole;2-Butyl-5-formylimidazole;2-n-Butyl-4-formylimidazole;2-n-Butyl-4-imidazolecarboxaldehyde;2-n-Butyl-5-imidazolecarboxaldehyde;2-butyl-1H-imidazole-5-carbaldehyde;1H-imidazole-5-carboxaldehyde, 2-butyl-;
- PSA 45.75000
- LogP 1.56480
Synthetic route
-
-
68283-19-2
2-butyl-5-hydroxymethyl-1H-imidazole

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; 2percent V/C In various solvent(s) at 105℃; under 750.075 Torr; for 6h; | 100% |
| With manganese(IV) oxide In dichloromethane Oxidation; |
-
-
7440-69-9
bismuth

-
-
7664-93-9
sulfuric acid

-
-
7440-44-0
pyrographite

-
-
68283-19-2
2-butyl-5-hydroxymethyl-1H-imidazole

-
-
7440-06-4
platinum

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 100% |
| With sodium hydroxide In water | 98.2% |
| With sodium hydroxide In methanol; water | 94.5% |

-
-
7440-69-9
bismuth

-
-
7440-44-0
pyrographite

-
-
68283-19-2
2-butyl-5-hydroxymethyl-1H-imidazole

-
-
108-10-1
4-methyl-2-pentanone

-
-
7440-06-4
platinum

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dodecane; water | 90% |

-
-
7440-69-9
bismuth

-
-
7440-44-0
pyrographite

-
-
68283-19-2
2-butyl-5-hydroxymethyl-1H-imidazole

-
-
108-10-1
4-methyl-2-pentanone

-
-
7440-06-4
platinum

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dodecane; water | 88.2% |

-
-
83857-96-9
2-n-butyl-4-chloro-1H-imidazol-5-carboxaldehyde

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-n-butyl-4-chloro-1H-imidazol-5-carboxaldehyde With hydrogen; triethylamine; palladium 10% on activated carbon In methanol at 20 - 25℃; under 2942.29 - 3677.86 Torr; for 8 - 10h; Stage #2: With hydrogenchloride In water at 25 - 30℃; pH=1.2; Stage #3: With sodium carbonate In water pH=6.8 - 7.5; Product distribution / selectivity; | 74% |
-
-
68283-19-2
2-n-butyl-4-(hydroxymethyl)imidazole

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide; bismuth(III) sulfate; dihydrogen peroxide; platinum on carbon In water at 20 - 62℃; for 3.5 - 4.5h; Product distribution / selectivity; | 61% |
-
-
50790-93-7
2-butyl-1H-imidazole

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2O 2: MnO2 / CH2Cl2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C 1.2: 2 h / 0 - 25 °C 2.1: n-butyllithium / tetrahydrofuran / -78 °C / Inert atmosphere 2.2: -78 - -50 °C / Inert atmosphere 2.3: 2 h / 0 - 25 °C View Scheme |
-
-
110-59-8
pentanonitrile

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 72 percent / hydrogen chloride / 18 h / 0 - 15 °C 2: 75 percent / NH3 / 60 °C / high pressure 3: MnO2 / CH2Cl2 View Scheme |

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; n-butyllithium In tetrahydrofuran; hexane; N,N-dimethyl-formamide |
-
-
148454-64-2
2-butyl-3-dimethylaminosulfonylimidazole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-butyl-3-dimethylaminosulfonylimidazole With n-butyllithium In tetrahydrofuran at -78℃; Inert atmosphere; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78 - -50℃; Inert atmosphere; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 0 - 25℃; for 2h; |
-
-
2417-72-3
Methyl 4-(bromomethyl)benzoate

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
A
-
133040-03-6
2-n-butyl-1-[(4-carbomethoxyphenyl)methyl]-1H-imidazol-5-carboxaldehyde

-
B
-
159708-89-1
methyl 4-[(2-butyl-4-formyl-1H-imidazol-1-yl)methyl]benzoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; Substitution; | A 27% B 54% |
| Stage #1: 2-butyl-1H-imidazole-5-carboxaldehyde With tetraethylammonium hydroxide In N,N-dimethyl-formamide for 1h; deprotonation; Stage #2: Methyl 4-(bromomethyl)benzoate In water; N,N-dimethyl-formamide at 0 - 20℃; for 3h; Alkylation; | A 40% B 54% |
| With sodium carbonate In N,N-dimethyl-formamide at 70℃; Alkylation; | |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |

-
-
114772-38-2
Methyl 4'-(bromomethyl)biphenyl-2-carboxylate

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
A
-
152147-96-1
2-butyl-1-<(2'-carbomethoxy<1,1'-biphenyl>-4-yl)methyl>-1H-imidazole-5-carboxaldehyde

-
B
-
470691-30-6
4'-(2-butyl-4-formyl-imidazol-1-ylmethyl)-biphenyl-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; | A 23% B 48% |

-
-
69460-11-3
(1R,2R,3R,5S)-(-)-Isopinocampheylamine

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R,3R,5S)-(-)-Isopinocampheylamine; 2-butyl-1H-imidazole-5-carboxaldehyde With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; for 1.5h; Inert atmosphere; Stage #2: With hydrogenchloride In ethyl acetate | 44% |
-
-
109-97-7
pyrrole

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| In propionic acid for 1h; Heating; | 26% |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
83857-96-9
2-n-butyl-4-chloro-1H-imidazol-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide Chlorination; |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
220579-46-4
4-[2-butyl-5-(2-ethoxycarbonyl-2-phenylsulfanyl-vinyl)-imidazol-1-ylmethyl]-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Na2CO3 / dimethylformamide / 70 °C 2.2: ethanol View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
220579-42-0
4-[2-butyl-4-(2-ethoxycarbonyl-2-phenylsulfanyl-vinyl)-imidazol-1-ylmethyl]-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Na2CO3 / dimethylformamide / 70 °C 2.2: ethanol View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: Na2CO3 / dimethylformamide / 70 °C 2.2: ethanol 3.1: NaOH / ethanol; H2O / Alkaline hydrolysis View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: Na2CO3 / dimethylformamide / 70 °C 2.2: ethanol 3.1: NaOH / ethanol; H2O / Alkaline hydrolysis View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
470691-32-8
4'-[5-((Z)-2-Azido-2-methoxycarbonyl-vinyl)-2-butyl-imidazol-1-ylmethyl]-biphenyl-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 23 percent / potassium carbonate / dimethylformamide / 25 °C 2: 42 percent / sodium; methanol / -15 °C View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
470691-34-0
4'-[4-((E)-2-Azido-2-methoxycarbonyl-vinyl)-2-butyl-imidazol-1-ylmethyl]-biphenyl-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 48 percent / potassium carbonate / dimethylformamide / 25 °C 2: 40 percent / sodium; methanol / -15 °C View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 23 percent / potassium carbonate / dimethylformamide / 25 °C 2: 42 percent / sodium; methanol / -15 °C 3: 32 percent / xylene / Heating View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
470691-39-5
methyl 2-(n-butyl)-1-[(2'-carbomethoxybiphenyl-4-yl)methyl]pyrrolo[3,2-d]imidazole-5-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 48 percent / potassium carbonate / dimethylformamide / 25 °C 2: 40 percent / sodium; methanol / -15 °C 3: 35 percent / xylene / Heating View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 27 percent / sodium carbonate / dimethylformamide / 24 h / 100 °C 2: 66 percent / NaOEt / tetrahydrofuran / 2 h / -10 °C View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 54 percent / sodium carbonate / dimethylformamide / 24 h / 100 °C 2: 40 percent / NaOEt / tetrahydrofuran / 2 h / -10 °C 3: 28 percent / xylene / 2 h / Heating View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 27 percent / sodium carbonate / dimethylformamide / 24 h / 100 °C 2: 66 percent / NaOEt / tetrahydrofuran / 2 h / -10 °C 3: 22 percent / xylene / 2 h / Heating View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
203204-02-8
α-azido-β-[2-butyl-1-(4-carbethoxybenzyl)imidazol-4-yl]acrylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 54 percent / sodium carbonate / dimethylformamide / 24 h / 100 °C 2: 40 percent / NaOEt / tetrahydrofuran / 2 h / -10 °C View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
133040-03-6
2-n-butyl-1-[(4-carbomethoxyphenyl)methyl]-1H-imidazol-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-chlorosuccinimide 2: K2CO3 / dimethylformamide 3: H2 / Pd/C View Scheme |
-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
133040-02-5
2-butyl-4-chloro-1-<(4-carbomethoxyphenyl)methyl>-1H-imidazole-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-chlorosuccinimide 2: K2CO3 / dimethylformamide View Scheme |
-
-
18997-19-8
Chloromethyl pivalate

-
-
133485-47-9
methyl (E)-3-<2-butyl-1-<(2-chlorophenyl)methyl>imidazol-5-yl>-2-<(2-thienyl)methyl>-2-propenoate

-
-
68282-49-5
2-butyl-1H-imidazole-5-carboxaldehyde

-
-
133486-33-6
2-n-butyl-1-pivalyloxymethylimidazol-5-carboxaldehyde

| Conditions | Yield |
|---|---|
| With manganese dioxide; potassium carbonate In N-methyl-acetamide |

2-Butyl-1H-imidazole-4-carbaldehyde Specification
The 1H-Imidazole-5-carboxaldehyde,2-butyl-, with the CAS registry number 68282-49-5, has the systematic name of 2-butyl-1H-imidazole-5-carbaldehyde. And the molecular formula of this chemical is C8H12N2O. It belongs to the product categories of Imidazol and Benzimidazole.
The physical properties of 1H-Imidazole-5-carboxaldehyde,2-butyl- are as following: (1)ACD/LogP: 0.65; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.63; (4)ACD/LogD (pH 7.4): 0.65; (5)ACD/BCF (pH 5.5): 1.74; (6)ACD/BCF (pH 7.4): 1.82; (7)ACD/KOC (pH 5.5): 51.19; (8)ACD/KOC (pH 7.4): 53.44; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 34.89 Å2; (13)Index of Refraction: 1.553; (14)Molar Refractivity: 44.34 cm3; (15)Molar Volume: 138.4 cm3; (16)Polarizability: 17.57×10-24cm3; (17)Surface Tension: 46.8 dyne/cm; (18)Density: 1.099 g/cm3; (19)Flash Point: 174.8 °C; (20)Enthalpy of Vaporization: 60.46 kJ/mol; (21)Boiling Point: 359.1 °C at 760 mmHg; (22)Vapour Pressure: 2.44E-05 mmHg at 25°C.
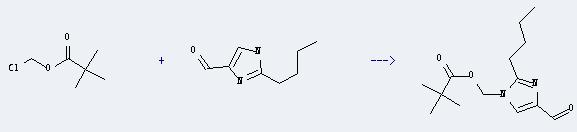
Uses of 1H-Imidazole-5-carboxaldehyde,2-butyl-: It can react with 2,2-dimethyl-propionic acid chloromethyl ester to produce 2,2-dimethyl-propionic acid 2-butyl-4-formyl-imidazol-1-ylmethyl ester. This reaction will need reagent K2CO3 and solvent dimethylformamide. The reaction time is 22 hours with temperature of 25°C, and the yield is about 99%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=Cc1cnc(n1)CCCC
(2)InChI: InChI=1/C8H12N2O/c1-2-3-4-8-9-5-7(6-11)10-8/h5-6H,2-4H2,1H3,(H,9,10)
(3)InChIKey: PTHGVOCFAZSNNA-UHFFFAOYAB
Related Products
- 2-Butyl-1H-imidazole-4-carbaldehyde
- 68282-52-0
- 68282-53-1
- 68283-19-2
- 6828-35-9
- 68284-01-5
- 68285-22-3
- 68285-23-4
- 68285-24-5
- 68285-25-6
- 68285-26-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View