-
Name
4-Nitrobenzyl chloride
- EINECS 202-822-7
- CAS No. 100-14-1
- Article Data90
- CAS DataBase
- Density 1.33 g/cm3
- Solubility Insoluble in water. Soluble in ethanol and diethyl ether. Easily soluble in benzene and acetone
- Melting Point 71 °C
- Formula C7H6ClNO2
- Boiling Point 292.515 °C at 760 mmHg
- Molecular Weight 171.583
- Flash Point 130.709 °C
- Transport Information UN 3261 8/PG 2
- Appearance Light yellow crystalline powder
- Safety 26-36/37/39-45-27
- Risk Codes 22-34-36
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Toluene, a-chloro-p-nitro- (8CI);1-(Chloromethyl)-4-nitrobenzene;4-(Chloromethyl)nitrobenzene;4-Chloromethyl-1-nitrobenzene;4-Nitrobenzyl chloride;4-Nitrophenylmethylchloride;NSC 9803;p-(Chloromethyl)nitrobenzene;p-Nitrobenzyl chloride;a-Chloro-4-nitrotoluene;a-Chloro-p-nitrotoluene;
- PSA 45.82000
- LogP 2.85680
Synthetic route

| Conditions | Yield |
|---|---|
| With indium(III) chloride; dimethylmonochlorosilane; benzil In dichloromethane at 20℃; for 24h; | 97% |
| With Ph3P(+)-N(CO2Et)-N(-)-CO2Et; N-methoxylamine hydrochloride In tetrahydrofuran at 0℃; for 0.0833333h; | 95% |
| Stage #1: 4-nitrobenzyl chloride With diisopropyl-carbodiimide; copper(l) chloride In tetrahydrofuran at 100℃; for 0.0833333h; microwave irradiation; Stage #2: With acetyl chloride In tetrahydrofuran at 150℃; for 0.0833333h; microwave irradiation; Further stages.; | 95% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel; tetramethylammonium nitrate for 0.0333333h; | 95% |
| With sulfuric acid; nitric acid In tetrachloromethane at 10℃; for 2h; | 82.4% |
| With sulfuric acid; nitric acid at 0 - 20℃; for 2h; | 45% |

| Conditions | Yield |
|---|---|
| With dichloromethylsilane; iron(III) chloride In 1,2-dimethoxyethane for 4h; Heating; | 95% |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / ethanol / 0 - 20 °C / Inert atmosphere 2: thionyl chloride / Reflux View Scheme |
-
-
619-73-8
4-nitrobenzyl chloride

-
-
75-36-5
acetyl chloride

-
A
-
619-90-9
4-nitrobenzyl acetate

-
B
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With 2,3-diethyl-2-cyclopropen-1-one In tert-butyl methyl ether at 20℃; for 4h; | A n/a B 90% |

-
-
191097-22-0
2-(2-tert-butyldimethylsiloxyethyl)-4H-benzo[1,4]oxazin-3-one

-
A
-
191096-97-6
3,4-Dihydro-2-(2-hydroxyethyl)-4-(4-nitrobenzyl)-3-oxo-2H-1,4-benzoxazine

-
B
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| A n/a B 89% |
-
-
14856-73-6
4-nitrobenzyl trimethylsilyl ether

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With 4-aminophenyl diphenylphosphinite; N-chloro-succinimide In dichloromethane for 3h; Heating; | 87% |
-
-
18483-99-3
2-(4-nitrobenzyloxy)tetrahydro-2H-pyran

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With 4-aminophenyl diphenylphosphinite; N-chloro-succinimide In dichloromethane for 5h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With tert-butylhypochlorite; Ag(Phen)2OTf In acetonitrile at 20℃; for 26h; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium chloride; tetrabutyl-ammonium chloride In water at 20℃; Irradiation; Green chemistry; | 82% |
| With N-chloro-succinimide; N-hydroxyphthalimide; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile at 80℃; for 16h; Time; Sealed tube; Inert atmosphere; | 67% |
| With Oxone; sodium chloride In chloroform; water at 20℃; for 18h; Solvent; Sealed tube; Irradiation; | 62% |

| Conditions | Yield |
|---|---|
| Stage #1: dichloromethane With n-butyllithium In tetrahydrofuran; hexane at -100℃; for 0.333333h; Inert atmosphere; Stage #2: nitrobenzene With N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran; hexane at -100℃; for 6h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; hexane; water at -100 - 20℃; Inert atmosphere; | 71% |
-
-
619-73-8
4-nitrobenzyl chloride

-
-
122-04-3
4-nitro-benzoyl chloride

-
A
-
3481-11-6
4'-(nitrobenzyl) 4-nitrobenzoate

-
B
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide In dichloromethane at 20℃; for 12h; | A n/a B 57% |


| Conditions | Yield |
|---|---|
| With 2-chloro-1,3-dimethylimidazolinium chloride; dimethyl sulfoxide; triethylamine In dichloromethane at 20℃; Chlorination; | A 13% B 43% |
-
-
292638-84-7
styrene

-
-
4025-75-6
(4-nitrophenyl)methanesulfonyl chloride

-
A
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| dichlorotris(triphenylphosphine)ruthenium(II) at 80℃; for 72h; | A 34% B 9% |


| Conditions | Yield |
|---|---|
| With gallium(III) trichloride; Hexamethyldisiloxane; copper dichloride In 1,2-dichloro-ethane at 20℃; for 5h; Sealed tube; Inert atmosphere; | 23% |
-
-
99-99-0
1-methyl-4-nitrobenzene

-
-
87-82-1
hexabromobenzene

-
A
-
100-14-1
4-nitrobenzyl chloride

-
B
-
100-11-8
1-bromomethyl-4-nitro-benzene

| Conditions | Yield |
|---|---|
| at 200℃; Einleiten von Chlor; |


| Conditions | Yield |
|---|---|
| With 1,4-dioxane; hydrogenchloride |

| Conditions | Yield |
|---|---|
| at 60℃; Rate constant; |
-
-
591-09-3
nitro acetate

-
-
100-44-7
benzyl chloride

-
A
-
100-14-1
4-nitrobenzyl chloride

-
B
-
612-23-7
2-nitrobenzyl chloride


| Conditions | Yield |
|---|---|
| With nitric acid | |
| With sulfuric acid; nitric acid at 5 - 10℃; | |
| With nitric acid; acetic anhydride at 30℃; |

| Conditions | Yield |
|---|---|
| With tributyltin chloride In benzene at 50℃; Thermodynamic data; Equilibrium constant; | 38 % Spectr. |
| With chloride In acetonitrile at 25℃; Rate constant; Equilibrium constant; other nucleophiles: Br(-); |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; thionyl chloride; 1,1,3,3-Tetramethyldisiloxane; zinc(II) iodide 1.) chlorotrimethylsilane, 1,1,3,3-tetramethyldisiloxane, thionyl chloride, ZnI2, -70 deg C, 1 h; 2.) DMF, reflux, 2 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
85313-83-3
N-chloro-S-(p-nitrobenzyl)-S-phenylsulfoximide

-
A
-
100-14-1
4-nitrobenzyl chloride

-
B
-
85313-79-7
S-(p-nitrobenzyl)-S-phenylsulfoximide

| Conditions | Yield |
|---|---|
| In ethanol; chloroform for 24h; Ambient temperature; |
-
-
39184-67-3
3-chloro-3-(p-nitrophenyl)diazirine

-
A
-
555-16-8
4-nitrobenzaldehdye

-
B
-
100-14-1
4-nitrobenzyl chloride

-
C
-
122-04-3
4-nitro-benzoyl chloride

| Conditions | Yield |
|---|---|
| With oxygen In 2,2,4-trimethylpentane Product distribution; Mechanism; Ambient temperature; Irradiation; |

-
-
83293-39-4
4-nitrobenzyl selenocyanate

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With chloride In acetonitrile at 25℃; Rate constant; Equilibrium constant; other nucleophiles: NCS(-), Br(-); |
-
-
81577-12-0
1-(1-Chloro-2,2,2-trifluoro-ethyl)-3-fluoro-benzene

-
A
-
128408-34-4
C8H5F4(1+)

-
B
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| at 69.85℃; Thermodynamic data; chloride-transfer; |

-
-
81577-14-2
1-Chloro-3-(1-chloro-2,2,2-trifluoro-ethyl)-benzene

-
A
-
128408-35-5
C8H5ClF3(1+)

-
B
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| at 69.85℃; Thermodynamic data; chloride-transfer; |

-
-
66650-27-9
1,1-dichloro-1λ4-benz[c][1,2]oxathiol-3-one

-
-
619-73-8
4-nitrobenzyl chloride

-
-
60-29-7
diethyl ether

-
A
-
100-14-1
4-nitrobenzyl chloride


| Conditions | Yield |
|---|---|
| With nitric acid |
-
-
7697-37-2
nitric acid

-
-
100-44-7
benzyl chloride

-
A
-
100-14-1
4-nitrobenzyl chloride

-
B
-
612-23-7
2-nitrobenzyl chloride

-
C
-
619-23-8
3-Nitrobenzyl chloride


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; benzylation; | 100% |
| With potassium carbonate In acetonitrile for 16h; Heating / reflux; | 70% |
| With ethanol |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
107-95-9
3-amino propanoic acid

-
-
294201-15-3
N-4-nitrobenzyl-β-alanine

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 20h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
-
-
638-14-2
2,4,6-trimethyl-[1,3,5]triazinane

-
-
100-14-1
4-nitrobenzyl chloride

-
-
64309-88-2
tris-(4-nitrobenzyl)amine

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 2h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; benzylation; | 99% |
| In ethanol; water for 1h; Heating; | 84% |
| With potassium carbonate In acetone for 46h; Heating; | 75% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
124-40-3
dimethyl amine

-
-
15184-96-0
4-(N,N-dimethylaminomethyl)nitrobenzene

| Conditions | Yield |
|---|---|
| In water; 4-methyl-2-pentanone at 20 - 35℃; | 99% |
| With ethanol at 100℃; | |
| In diethyl ether |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; benzylation; | 99% |
| With potassium carbonate In xylene for 1h; Heating; | 59% |
| With triethylamine In dichloromethane at 20℃; | |
| With sodium carbonate In chloroform at 65℃; for 18h; | 40.4 g |
| In toluene at 90℃; for 5h; |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; cetyltributylphosphonium bromide In water; toluene at 40℃; for 0h; Product distribution; | 99% |
| With phosphonic Acid; iodine In benzene at 100℃; for 36h; Inert atmosphere; | 42% |
| With tri-n-butyl-tin hydride; 2,2'-azobis(isobutyronitrile) In toluene for 3h; Mechanism; Irradiation; | 24% |
-
-
35386-24-4
1-(2-Methoxyphenyl)piperazine

-
-
100-14-1
4-nitrobenzyl chloride

-
-
270062-75-4
1-(4-nitrobenzyl)-4-(2-methoxyphenyl)piperazine

| Conditions | Yield |
|---|---|
| With silica gel for 0.0833333h; Alkylation; Microwave irradiation; | 99% |
| With potassium carbonate In butanone at 80℃; for 24h; | 89% |

| Conditions | Yield |
|---|---|
| With silica gel for 0.0833333h; Alkylation; Microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic trihydrate In toluene at 80℃; for 0.5h; Solvent; Temperature; Suzuki-Miyaura Coupling; Inert atmosphere; | 99% |
| With sodium carbonate; trans-PdBr(N-Succ)(PPh3)2 In tetrahydrofuran at 80℃; for 3h; Suzuki-Miyaura cross-coupling; | 93% |
| With cis,cis,cis-tetrakis[(diphenylphosphanyl)methyl]cyclopentane; potassium carbonate; bis(η3-allyl-μ-chloropalladium(II)) In xylene at 130℃; for 20h; Syzuki cross-coupling; | 83% |
| With potassium carbonate; N,N-dimethyl-formamide; palladium dichloride In water at 90℃; for 1h; Suzuki Coupling; | 77% |
| With water; sodium carbonate; triphenylphosphine; palladium dichloride In tetrahydrofuran at 40℃; for 24h; Suzuki-Miyaura Coupling; Sealed tube; | 54 %Spectr. |

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With potassium iodide In acetone at 20℃; for 1h; | 99% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
95668-86-3
1-(azidomethyl)-2-nitrobenzene

| Conditions | Yield |
|---|---|
| With sodium azide; ammonium chloride In water at 70℃; for 48h; | 99% |

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium azide; triethylamine at 100℃; for 0.0833333h; Microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; dimethyl selenoxide; tetrabutylammomium bromide In 1,2-dimethoxyethane for 4h; Heating; | 98% |
| With bismuth(III) nitrate; tetrabutyl ammonium fluoride at 100℃; for 2.7h; | 95% |
| With 1-dodecyl-3-methylimidazolium iron chloride; periodic acid at 30℃; for 2.5h; | 90% |
-
-
106-42-3
para-xylene

-
-
100-14-1
4-nitrobenzyl chloride

-
-
85716-73-0
1,4-dimethyl-2-(4-nitrobenzyl)benzene

| Conditions | Yield |
|---|---|
| With graphite-supported iron oxide nanoparticles for 4h; Reflux; | 98% |
| With iron(III) chloride for 6.5h; Heating; | 83% |
-
-
84624-27-1
Boc-Lys(Fmoc)-OH

-
-
100-14-1
4-nitrobenzyl chloride

-
-
134653-28-4
Boc-Lys(Fmoc) p-nitrobenzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; potassium iodide In N,N-dimethyl-formamide at 25℃; for 5h; | 98% |
-
-
136918-14-4
phthalimide

-
-
100-14-1
4-nitrobenzyl chloride

-
-
62133-07-7
2-(4-nitrobenzyl)-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 8h; Alkylation; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 89% |
| With di-μ-hydroxo-bis[(N,N,N′,N′-tetramethylethylenediamine)copper(II)] chloride; caesium carbonate In acetonitrile at 20℃; for 4h; | 85% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
50793-60-7
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isoselenuronium bromide

-
-
1383478-47-4
4-nitrobenzyl 2,3,4,6-tetra-O-acetyl-1-seleno-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; | 98% |
-
-
4394-85-8
4-morpholinecarboxaldehyde

-
-
100-14-1
4-nitrobenzyl chloride

-
-
6425-46-3
4-(4-nitrobenzyl)morpholine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 50℃; for 3h; Green chemistry; | 98% |
| With NHC-Pd(II)-Im; sodium hydroxide In water at 50℃; for 3h; Inert atmosphere; Schlenk technique; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 50℃; for 3h; Green chemistry; | 98% |
-
-
1315320-86-5
5-acetyl-6-methyl-4-phenyl-1-(prop-2-yn-1-yl)-3,4-dihydropyrimidin-2(1H)-one

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium azide; triethylamine at 100℃; for 0.05h; Microwave irradiation; | 98% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
26798-33-4
(p-nitrophenyl)methanethiol

| Conditions | Yield |
|---|---|
| With hydrosulfide exchange resin In acetonitrile at 25℃; for 0.25h; | 97% |
| With ammonium sulfide | |
| (i) thiourea, (ii) aq. NaOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 80℃; for 12h; | 97% |

-
-
100-14-1
4-nitrobenzyl chloride

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium azide; triethylamine at 100℃; for 0.05h; Microwave irradiation; | 97% |
-
-
33252-63-0
5-(trifluoromethyl)-2(1H)-pyridone

-
-
100-14-1
4-nitrobenzyl chloride

-
-
923688-20-4
5-trifluoromethyl-(1-(4-nitro)benzyl)pyridine-2(1H)-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 130℃; for 4h; | 96.9% |

| Conditions | Yield |
|---|---|
| With sodium dodecyl-sulfate; sodium hydrogencarbonate In water at 80℃; for 1.5h; | 96% |
| With sodium carbonate |
4-Nitrobenzyl chloride Consensus Reports
4-Nitrobenzyl chloride Specification
The 4-Nitrobenzyl chloride, with the CAS registry number 100-14-1, is also known as p-Nitrobenzyl chloride. It belongs to the product category of Aromatic Halides (substituted). Its EINECS number is 202-822-7. This chemical's molecular formula is C7H6ClNO2 and molecular weight is 171.58. What's more, its systematic name is 1-(Chloromethyl)-4-nitrobenzene. Its classification code is Mutation data. This chemical should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides, heat and fire. It is used as chemical reagent and it is also used in organic synthesis.
Physical properties of 4-Nitrobenzyl chloride are: (1)ACD/LogP: 2.355; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.36; (4)ACD/LogD (pH 7.4): 2.36; (5)ACD/BCF (pH 5.5): 36.27; (6)ACD/BCF (pH 7.4): 36.27; (7)ACD/KOC (pH 5.5): 454.94; (8)ACD/KOC (pH 7.4): 454.94; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 45.82 Å2; (13)Index of Refraction: 1.574; (14)Molar Refractivity: 42.564 cm3; (15)Molar Volume: 128.97 cm3; (16)Polarizability: 16.874×10-24cm3; (17)Surface Tension: 47.2 dyne/cm; (18)Density: 1.33 g/cm3; (19)Flash Point: 130.709 °C; (20)Enthalpy of Vaporization: 51.071 kJ/mol; (21)Boiling Point: 292.515 °C at 760 mmHg; (22)Vapour Pressure: 0.003 mmHg at 25°C.
Preparation: it is prepared by reaction of (4-nitro-phenyl)-methanol. The reaction needs reagents Ph3P(+)-N(CO2Et)-N(-)-CO2Et, MeONH2·HCl and solvent tetrahydrofuran at the temperature of 0 °C. The yield is about 95%.

Uses of 4-Nitrobenzyl chloride: it can be used as pharmaceutical intermediates and for organic synthesis. Besides, it is used to produce 1-(4-nitro-benzyl)-pyrrolidine by benzylation reaction with pyrrolidine. The reaction occurs with solvent tetrahydrofuran at the temperature of 20 °C. The yield is about 99%.
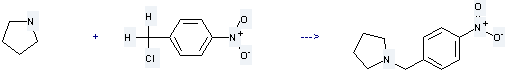
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes and it is harmful if swallowed. It can cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. After using it, you should take off immediately all contaminated clothing. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: ClCc1ccc([N+]([O-])=O)cc1
(2)Std. InChI: InChI=1S/C7H6ClNO2/c8-5-6-1-3-7(4-2-6)9(10)11/h1-4H,5H2
(3)Std. InChIKey: KGCNHWXDPDPSBV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LCLo | inhalation | 280mg/m3/4H (280mg/m3) | Toxicologist. Vol. 4, Pg. 66, 1984. | |
| rat | LD50 | oral | 1809mg/kg (1809mg/kg) | Inhalation Toxicology. Vol. 3, Pg. 265, 1991. |
Related Products
- 4-Nitrobenzyl [2R-(2alpha,5beta,6alpha,7beta)]-3-methylene-8-oxo-7-(phenoxyacetamido)-5-thia-1-azabicyclo[4.2.0]octane-2-carboxylate 5-oxide
- 4-Nitrobenzyl 2-diazoacetoacetate
- 4-NITROBENZYL ACETATE
- 4-Nitrobenzyl alcohol
- 4-Nitrobenzyl bromide
- 4-Nitrobenzyl chloride
- 4-Nitrobenzyl chloroformate
- 4-Nitrobenzyl hydrogen malonate
- 4-Nitrobenzylamine
- 4-Nitrobenzylamine hydrochloride
- 1001412-59-4
- 1001419-41-5
- 100142-85-6
- 100145-04-8
- 100-15-2
- 1001-52-1
- 1001-53-2
- 100157-55-9
- 100158-38-1
- 1001-58-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View