-
Name
7-Octyn-1-ol
- EINECS
- CAS No. 871-91-0
- Article Data66
- CAS DataBase
- Density 0.889 g/cm3
- Solubility
- Melting Point -39°C (estimate)
- Formula C8H14O
- Boiling Point 191.5 °C at 760 mmHg
- Molecular Weight 126.199
- Flash Point 116.3 °C
- Transport Information
- Appearance
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols
- Synonyms 8-Hydroxy-1-octyne;Oct-7-yn-1-ol;Octane-7-yne-1-ol;
- PSA 20.23000
- LogP 1.56240
Synthetic route
-
-
14916-80-4
oct-3-yn-1-ol

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydride In ethylenediamine; mineral oil at 45 - 65℃; | 100% |
| With potassium hydride; Trimethylenediamine for 18h; Ambient temperature; | 97% |
| With potassium tert-butylate; lithium In ethylenediamine at 25℃; for 3h; | 96% |
-
-
10297-09-3
oct-7-ynoic acid

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 3h; Inert atmosphere; | 96% |
| Multi-step reaction with 2 steps 1: diethyl ether 2: LiAlH4 / diethyl ether / 2 h / 0 °C View Scheme | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 3h; |
-
-
20739-58-6
2-octyn-1-ol

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydride; Trimethylenediamine In hexane; mineral oil at 0 - 20℃; for 3h; | 91% |
| With potassium salt of 1,3-diaminopropane In ammonia | 90% |
| With sodium hydride; ethylenediamine | 86% |
-
-
34126-19-7
4-octyne-1-ol

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With sodium amide; Trimethylenediamine at 80℃; for 2.5h; | 90% |
| With sodium amide; Trimethylenediamine at 80℃; for 4h; | 72.1% |
-
-
16695-31-1
1-(2-Tetrahydropyranyloxy)-7-octyne

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 144h; | 80% |
-
-
52517-97-2
1-triphenylmethyloxyoct-7-yne

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid In dichloromethane | 79% |
-
-
119837-87-5
8-(t-Butyldimethylsilyloxy)-1-octyne

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran; water; acetic acid at 80 - 90℃; for 2h; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: ethylenediamine With sodium hydride at 0 - 60℃; for 1h; Inert atmosphere; Stage #2: oct-3-yn-1-ol at 60℃; for 1h; Inert atmosphere; | 58% |
-
-
24612-83-7
1-octene-4-yne

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| (i) 9-bora-bicyclo<3.3.1>nonane, THF, (ii) propane-1,3-diamine , benzene, (iii) H2O2, aq. NaOH; Multistep reaction; |
-
-
18458-50-9
7-octynoic acid methyl ester

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; for 2h; Yield given; | |
| With lithium borohydride In tetrahydrofuran Reduction; |
-
-
188579-46-6
1-ethoxyethyl 7-octynyl ether

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: n-BuLi / tetrahydrofuran; hexamethylphosphoric acid triamide 2: KNH(CH2)3NH2 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) Li, NH3, Fe(NO3)3 / 1.) -78 deg C, 30 min, 2.) -78 deg C, 1.5 h 2: 82 percent / Li, 1,2-diaminopropane, tert-BuOK / 1 h View Scheme |
-
-
28659-22-5
6-(tetrahydro-2H-pyranyloxy)hexan-1-ol

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 84 percent / pyridine / CH2Cl2 / 16 h / Ambient temperature 2: 46 percent / I2, 1,2-bis(diphenylphosphino)ethane / CH2Cl2 / 4 h / Ambient temperature 3: 34 percent / dimethylsulfoxide / 2 h / Ambient temperature 4: 79 percent / trifluoroacetic acid, H2O / CH2Cl2 View Scheme |
-
-
158576-27-3
1-iodo-6-triphenylmethyloxyhexane

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 34 percent / dimethylsulfoxide / 2 h / Ambient temperature 2: 79 percent / trifluoroacetic acid, H2O / CH2Cl2 View Scheme |
-
-
158576-23-9
1-tetrahydropyranyloxy-6-triphenylmethyloxyhexane

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 46 percent / I2, 1,2-bis(diphenylphosphino)ethane / CH2Cl2 / 4 h / Ambient temperature 2: 34 percent / dimethylsulfoxide / 2 h / Ambient temperature 3: 79 percent / trifluoroacetic acid, H2O / CH2Cl2 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / LiNH2 / liquid ammonia / 3 h 2: 90 percent / NaNH2, H2N(CH2)3NH2 / 2.5 h / 80 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NH3 / tetrahydrofuran / 15 h 2: diethyl ether 3: LiAlH4 / diethyl ether / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / tetrahydrofuran / 0 - 20 °C / Inert atmosphere 2: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 0 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: n-butyllithium; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / tetrahydrofuran / 1.25 h / -78 °C 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 3 h / 20 °C 3: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 0 - 20 °C View Scheme |
-
-
59431-24-2
1-(tert-butyl-dimethylsilyloxy)-6-chloro-hexane

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodiumiodideo / acetone / 36 h / Heating 2: dimethylformamide / 2 h / Ambient temperature 3: 75 percent / water / acetic acid; tetrahydrofuran; H2O / 2 h / 80 - 90 °C View Scheme |
-
-
103483-32-5
1-iodo-6-(tert-butyldimethylsiloxy)hexane

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide / 2 h / Ambient temperature 2: 75 percent / water / acetic acid; tetrahydrofuran; H2O / 2 h / 80 - 90 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 84 percent / LiNH2, liq. NH3 2: 78 percent / t-BuOK, Li, H2N-(CH2)3-NH2 View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / LiNH2 / liquid ammonia 2: 90 percent / K(1+)*NH(1-)(CH2)3NH2 / liquid ammonia View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / LiNH2 / liquid ammonia 2: 85 percent / H2N(CH2)3NH2, Li, t-BuOK View Scheme | |
| Multi-step reaction with 2 steps 1: ammonia; Iron(III) nitrate nonahydrate; lithium / tetrahydrofuran / -78 - 20 °C 2: sodium hydride; ethylenediamine / mineral oil / 0 - 70 °C View Scheme |
-
-
1013026-76-0
8-(trimethylsilyl)-oct-7-ynoic acid

-
-
871-91-0
oct-7-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 3 h / 20 °C 2: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 0 - 20 °C View Scheme |
-
-
871-91-0
oct-7-yn-1-ol

-
-
81216-13-9
8-bromo-1-octyne

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane for 1h; Inert atmosphere; Reflux; | 100% |
| With N-Bromosuccinimide; triphenylphosphine In dichloromethane at 0 - 20℃; Inert atmosphere; | 60% |
| With phosphorus tribromide at 0℃; | |
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 0 - 20℃; for 2h; | |
| With carbon tetrabromide; triphenylphosphine In dichloromethane for 1h; Reflux; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 35℃; for 12h; | 99% |
| With 1H-imidazole; dmap In tetrahydrofuran at 0℃; for 1.08333h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane at -5 - 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: oct-7-yn-1-ol With n-butyllithium In tetrahydrofuran; hexane at -78 - 0℃; for 0.333333h; Stage #2: chloro-trimethyl-silane In tetrahydrofuran; hexane at -78 - 20℃; | 100% |
| Stage #1: oct-7-yn-1-ol With n-butyllithium In tetrahydrofuran at -78℃; for 1.5h; Inert atmosphere; Stage #2: chloro-trimethyl-silane In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 90% |
| Stage #1: oct-7-yn-1-ol With n-butyllithium Stage #2: chloro-trimethyl-silane | 83% |
| Stage #1: oct-7-yn-1-ol With n-butyllithium In tetrahydrofuran at 0℃; for 0.666667h; Stage #2: chloro-trimethyl-silane In tetrahydrofuran at 0 - 20℃; for 2h; | 80% |
| Stage #1: oct-7-yn-1-ol With methylmagnesium bromide In tetrahydrofuran; diethyl ether at 0 - 20℃; for 16h; Inert atmosphere; Stage #2: chloro-trimethyl-silane In tetrahydrofuran; diethyl ether for 8h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| camphor-10-sulfonic acid In dichloromethane at 0 - 20℃; | 99% |
| With toluene-4-sulfonic acid | 96% |
| With toluene-4-sulfonic acid In dichloromethane for 8h; Ambient temperature; | 85% |
| With toluene-4-sulfonic acid In dichloromethane at 0 - 20℃; for 0.25h; | 84% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
139140-55-9
7-octyn-1-yl tert-butyldiphenylsilyl ether

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 5h; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide for 2h; Ambient temperature; | 93% |
| With 1H-imidazole In N,N-dimethyl-formamide for 2h; Ambient temperature; | 93% |
| Yield given; |
-
-
1192358-22-7
N-(2-azidoethyl)-4-pentylbenzamide

-
-
871-91-0
oct-7-yn-1-ol

-
-
1430850-09-1
N-(2-(4-(6-hydroxyhexyl)-1H-1,2,3-triazol-1-yl)ethyl)-4-pentylbenzamide

| Conditions | Yield |
|---|---|
| With copper(II) sulfate; sodium L-ascorbate In dichloromethane; water; tert-butyl alcohol at 20℃; for 16h; Huisgen Cycloaddition; | 99% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 21h; | 98% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
82402-15-1
oct-6-yn-1-ol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 80℃; Rearrangement; | 96% |
| With potassium tert-butylate In dimethyl sulfoxide at 80℃; for 0.0833333h; | 93% |
| With potassium tert-butylate In dimethyl sulfoxide at 80 - 83℃; for 0.0833333h; | 84.5% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
333754-13-5
8-iodo-7-octyn-1-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; iodine In methanol; water at 20℃; for 3h; | 95% |
| With potassium hydroxide; iodine In methanol at 20℃; for 3h; | 95% |
| Stage #1: oct-7-yn-1-ol With potassium hydroxide In methanol; water at 0℃; for 0.166667h; Stage #2: With iodine In methanol; water at 20℃; for 3.5h; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: oct-7-yn-1-ol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: benzyl bromide In tetrahydrofuran; mineral oil at 0 - 20℃; for 2h; | 95% |
| Stage #1: oct-7-yn-1-ol With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; for 3h; Inert atmosphere; | 92% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
13175-44-5
oct-7-en-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; Lindlar's catalyst In methanol for 0.75h; | 94% |

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; dimethyl sulfoxide; triethylamine In dichloromethane at -60 - 0℃; Swern oxidation; | 94% |
| Stage #1: oct-7-yn-1-ol With phosgene; dimethyl sulfoxide In dichloromethane at -78℃; for 0.25h; Swern Oxidation; Inert atmosphere; Stage #2: With triethylamine In dichloromethane; dimethyl sulfoxide at 20℃; for 4h; Inert atmosphere; Further stages; | 88% |
| With sulfur trioxide pyridine complex; dimethyl sulfoxide; triethylamine In dichloromethane Inert atmosphere; | 86% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
197218-85-2
ethyl (2Z)-4,4,4-trifluoro-3-iodobut-2-enoate

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine for 24h; Ambient temperature; | 93% |
-
-
868132-31-4
10-((tert-butyldiphenylsilyl)oxy)decanoic acid

-
-
871-91-0
oct-7-yn-1-ol

-
-
868132-33-6
10-(tert-butyldiphenylsilanyloxy)decanoic acid oct-7-ynyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In diethyl ether at 20℃; for 16h; | 93% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
10297-09-3
oct-7-ynoic acid

| Conditions | Yield |
|---|---|
| With Jones reagent | 92% |
| With Iron(III) nitrate nonahydrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; sodium chloride In 1,2-dichloro-ethane at 20℃; for 20h; Schlenk technique; | 85% |
| With Iron(III) nitrate nonahydrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium chloride; oxygen In 1,2-dichloro-ethane at 25℃; for 12h; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; copper(I) iodide; triethylamine In dichloromethane; water; argon | 91% |


| Conditions | Yield |
|---|---|
| Stage #1: oct-7-yn-1-ol With hydroxylamine hydrochloride; ethylamine; copper(l) chloride In methanol; water at 0℃; Inert atmosphere; Stage #2: 1-nonynyl bromide In methanol; water at 0 - 20℃; Inert atmosphere; | 91% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
154533-69-4
8-Azido-oct-1-yne

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine; triphenylphosphine; diethylazodicarboxylate In benzene at 0 - 20℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine Inert atmosphere; | 89% |
| With pyridine at 20℃; | 81% |
| With pyridine at -10 - 20℃; for 2h; Tosylation; | 77% |

| Conditions | Yield |
|---|---|
| With Montmorillonite KSF clay; copper(I) bromide for 0.0833333h; microwave irradiation; | 89% |

-
-
871-91-0
oct-7-yn-1-ol

-
-
199169-51-2
10-bromo-8-decynoic acid methyl ester

-
-
262603-16-7
methyl 18-hydroxyoctadeca-8,11-diynoate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 8h; cross-coupling; | 87% |
-
-
871-91-0
oct-7-yn-1-ol

-
-
60754-50-9
8-iodo-1-octyne

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; triphenylphosphine In dichloromethane at 22℃; for 1.08333h; | 85.1% |
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere 2: sodium iodide / acetone / 60 °C View Scheme |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 20℃; for 2.5h; | 81% |
7-Octyn-1-ol Specification
The 7-Octyn-1-ol with CAS registry number of 871-91-0 is also known as Octane-7-yne-1-ol. The IUPAC name is oct-7-yn-1-ol. In addition, the formula is C8H14O and the molecular weight is 126.2. This chemical is flammable. During using it, keep away from sources of ignition.
Physical properties about 7-Octyn-1-ol are: (1)ACD/LogP: 1.65; (2)ACD/LogD (pH 5.5): 1.65; (3)ACD/LogD (pH 7.4): 1.65; (4)ACD/BCF (pH 5.5): 10.55; (5)ACD/BCF (pH 7.4): 10.55; (6)ACD/KOC (pH 5.5): 187.92; (7)ACD/KOC (pH 7.4): 187.92; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 6; (11)Polar Surface Area: 9.23 Å2; (12)Index of Refraction: 1.454; (13)Molar Refractivity: 38.47 cm3; (14)Molar Volume: 141.8 cm3; (15)Polarizability: 15.25x10-24cm3; (16)Surface Tension: 35.4 dyne/cm; (17)Density: 0.889 g/cm3; (18)Flash Point: 116.3 °C; (19)Enthalpy of Vaporization: 49.77 kJ/mol; (20)Boiling Point: 191.5 °C at 760 mmHg; (21)Vapour Pressure: 0.138 mmHg at 25 °C.
Preparation of 7-Octyn-1-ol: it is prepared by reaction of oct-3-yn-1-ol. This reaction needs reagents 1,3-diaminopropane, NaH at 80 °C for 2 hours.

Uses of 7-Octyn-1-ol: it can be used to produce oct-7-ynoic acid. This reaction occurs with reagent Jones reagent and solvent acetone at ambient temperature for 30 minutes. The yield is about 57%.
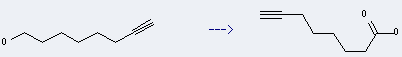
You can still convert the following datas into molecular structure:
(1)SMILES: C#CCCCCCCO
(2)InChI: InChI=1/C8H14O/c1-2-3-4-5-6-7-8-9/h1,9H,3-8H2
(3)InChIKey: ATCNYMVVGBLQMQ-UHFFFAOYAN
(4)Std. InChI: InChI=1S/C8H14O/c1-2-3-4-5-6-7-8-9/h1,9H,3-8H2
(5)Std. InChIKey: ATCNYMVVGBLQMQ-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View