-
Name
ETHYL DICHLOROACETATE
- EINECS 208-611-6
- CAS No. 535-15-9
- Article Data57
- CAS DataBase
- Density 1.295 g/cm3
- Solubility with ethanol, ethyl ether immiscible, insoluble in water
- Melting Point
- Formula C4H6Cl2O2
- Boiling Point 151.1 °C at 760 mmHg
- Molecular Weight 156.996
- Flash Point 55.6 °C
- Transport Information UN 2810
- Appearance colorless liquid
- Safety 23-26-36
- Risk Codes 20/21-36/37
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Aceticacid, dichloro-, ethyl ester (6CI,7CI,8CI,9CI);Dichloroacetic acid ethylester;Ethyl 2,2-dichloroacetate;Ethyl dichloroacetate;NSC 27788;NSC 6748;Acetic acid, dichloro-, ethyl ester;AC1L1VZN;35820_ALDRICH;
- PSA 26.30000
- LogP 1.35320
Synthetic route

| Conditions | Yield |
|---|---|
| at 0℃; | 96% |
-
-
54567-92-9
1,1,1-triethoxy-2,2-dichloroethane

-
A
-
64-17-5
ethanol

-
B
-
141-78-6
ethyl acetate

-
C
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With acetic acid Heating; | A n/a B n/a C 95% |

-
-
64-17-5
ethanol

-
-
1768-31-6
pentachloroacetone

-
A
-
67-66-3
chloroform

-
B
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Heating; | A 9.2 g B 82.2% |


| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In N,N-dimethyl acetamide at 25℃; under 760.051 Torr; for 1h; chemoselective reaction; | 79% |
| With 10% Pt/activated carbon; hydrogen In N,N-dimethyl acetamide at 25℃; under 760.051 Torr; for 1h; chemoselective reaction; | 79% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 28℃; for 72h; Irradiation; Inert atmosphere; | 70% |
-
-
64-17-5
ethanol

-
-
87651-34-1
pentachloro-2-(trimethylsiloxy)propene

-
A
-
67-66-3
chloroform

-
B
-
1825-62-3
ethyl trimethylsilyl ether

-
C
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With triethylamine for 8h; Heating; Yields of byproduct given; | A n/a B n/a C 71% |
| With triethylamine for 8h; Heating; Yield given; | A n/a B n/a C 71% |

-
-
86164-39-8
2,2-dichloro-3-oxo-propionic acid ethyl ester

-
A
-
535-15-9
ethyl 1,1-dichloroacetate

-
B
-
103-70-8
Formanilid

| Conditions | Yield |
|---|---|
| With aniline In tetrachloromethane for 12h; Ambient temperature; | A 50% B 66% |
-
-
86164-39-8
2,2-dichloro-3-oxo-propionic acid ethyl ester

-
-
62-53-3
aniline

-
A
-
535-15-9
ethyl 1,1-dichloroacetate

-
B
-
103-70-8
Formanilid

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 12h; Ambient temperature; | A 50% B 66% |

-
-
64-17-5
ethanol

-
-
79-01-6
Trichloroethylene

-
-
141-78-6
ethyl acetate

-
A
-
354-14-3
freon-121

-
B
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With chlorine monofluoride 1.) Freon 113; 2.) 20 - 25 deg C, 30 min.; Yield given. Multistep reaction; | A 65% B n/a |

-
-
79-43-6
dichloro-acetic acid

-
-
1569262-64-1
ethyl N-tert-butyl-4-nitrobenzenesulfonimidate

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| In dichloromethane Heating; | 58% |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; copper(II) sulfate; sodium sulfate for 2h; Heating; | 40% |
| With hydrogenchloride | |
| With sulfuric acid unter wiederholtem Abdestillieren des entstandenen Wassers mit Benzol; |

| Conditions | Yield |
|---|---|
| at 100 - 120℃; |

| Conditions | Yield |
|---|---|
| at 25℃; unter Lichtausschluss; |

-
-
64-17-5
ethanol

-
-
1768-31-6
pentachloroacetone

-
-
555-75-9
aluminum ethoxide

-
A
-
67-66-3
chloroform

-
B
-
535-15-9
ethyl 1,1-dichloroacetate


| Conditions | Yield |
|---|---|
| With aluminum ethoxide |

| Conditions | Yield |
|---|---|
| at 150℃; |
-
-
64-17-5
ethanol

-
-
24298-56-4
2,2-diacetoxy-1,1,1-trichloro-ethane

-
-
151-50-8
potassium cyanide

-
A
-
141-78-6
ethyl acetate

-
B
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
64-17-5
ethanol

-
-
24298-56-4
2,2-diacetoxy-1,1,1-trichloro-ethane

-
-
151-50-8
potassium cyanide

-
-
535-15-9
ethyl 1,1-dichloroacetate


| Conditions | Yield |
|---|---|
| With sulfuric acid; potassium chloride |

| Conditions | Yield |
|---|---|
| beim Chlorieren; |
-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With chlorine at 25℃; dann Behandeln mit Wasser; |

-
-
64-17-5
ethanol

-
-
631-61-8
ammonium acetate

-
-
86164-43-4
2-acetoxy-3,3,3-trichloro-propionitrile

-
A
-
683-72-7
dichloroacetamide

-
B
-
535-15-9
ethyl 1,1-dichloroacetate


-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| at 18 - 25℃; Yield given; |
-
-
54567-93-0
1,1-dichloro-2,2-diethoxy-ethene

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In [D3]acetonitrile |
-
-
515-84-4
Ethyl trichloroacetate

-
A
-
74-87-3
methylene chloride

-
B
-
75-09-2
dichloromethane

-
C
-
105-39-5
chloroacetic acid ethyl ester

-
D
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With hydrogen cation; tetranormalbutyl ammonium fluoride for 0.166667h; Product distribution; Ambient temperature; equilibrium; products determined by GC-MS; |

-
-
515-84-4
Ethyl trichloroacetate

-
A
-
3875-96-5
tetrachloro-succinic acid diethyl ester

-
B
-
15649-41-9
diethyl (Z)-2,3-dichlorobutenedioate

-
C
-
15649-40-8
diethyl 2,3-dichlorofumarate

-
D
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With iron(II) chloride In acetonitrile at 25 - 82℃; Rate constant; Thermodynamic data; Product distribution; ΔH(excit.), ΔS(excit.), var. reagent conc., var. reaction time; |

-
-
938-81-8
N-nitrosoacetanilide

-
-
112260-88-5
ethyl dichlorothionoacetate

-
A
-
28663-68-5
ethyl (phenylhydrazono)chloroacetate

-
B
-
112260-89-6
4-Chloro-2-phenyl-2H-[1,2,3]thiadiazol-5-one

-
C
-
882-33-7
diphenyldisulfane

-
D
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 20h; Yield given. Yields of byproduct given; |

-
-
112260-88-5
ethyl dichlorothionoacetate

-
A
-
28663-68-5
ethyl (phenylhydrazono)chloroacetate

-
B
-
112260-89-6
4-Chloro-2-phenyl-2H-[1,2,3]thiadiazol-5-one

-
C
-
882-33-7
diphenyldisulfane

-
D
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With N-nitrosoacetanilide In acetone at 20℃; for 20h; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In benzene at 80℃; for 10h; other esters, other solvents, other temperatures and reaction times; | 100% |
| With bis(tri-n-butyltin)oxide In benzene at 80℃; for 10h; | 100% |
| With 2,2'-azobis(isobutyronitrile); bis(tri-n-butyltin)oxide In benzene at 80℃; for 1.5h; | 100 % Spectr. |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |
-
-
66-25-1
hexanal

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
111055-67-5
2-Chloro-3-pentyl-oxirane-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In diethyl ether 0-5 deg C, 1 h; reflux, 3 h; | 98% |
| With sodium ethanolate 1.) ether, 30 min, 2.) ether, reflux, 3 h; Multistep reaction; | |
| With base In diethyl ether Darzens condensation; |
-
-
75-08-1
ethanethiol

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
20461-95-4
Bis(ethylthio)essigsaeure-ethylester

| Conditions | Yield |
|---|---|
| With potassium carbonate; Aliquat 336 In toluene for 8h; Ambient temperature; | 98% |
-
-
590-86-3
isovaleraldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
111055-66-4
2-Chloro-3-isobutyl-oxirane-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In diethyl ether 0-5 deg C, 1 h; reflux, 3 h; | 98% |
| With base In diethyl ether Darzens condensation; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In N,N-dimethyl-formamide | 97% |
-
-
895570-99-7
(2R,3R)-2-benzylamino-3-(4-methylsulfonylphenyl)-propane-1,3-diol

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
15318-45-3
thiamphenicol

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3R)-2-benzylamino-3-(4-methylsulfonylphenyl)-propane-1,3-diol With hydrogenchloride; hydrogen; palladium on activated charcoal In ethanol at 20℃; for 2h; Stage #2: ethyl 1,1-dichloroacetate With triethylamine In methanol at 30℃; for 6h; Further stages.; | 96% |
-
-
895571-10-5
(1R,2S)-1-(4-methylsulfonylphenyl)-2-benzylamino-3-fluoro-1-propanol

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
73231-34-2
Florfenicol

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2S)-1-(4-methylsulfonylphenyl)-2-benzylamino-3-fluoro-1-propanol With sulfuric acid; hydrogen; palladium on activated charcoal In ethanol at 20℃; for 2h; Stage #2: ethyl 1,1-dichloroacetate With triethylamine In methanol at 30℃; for 6h; Further stages.; | 96% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In acetonitrile Reagent/catalyst; Solvent; Reflux; | 93.98% |

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 12h; | 93% |
-
-
126227-04-1
(2-Benzylamino-ethyl)-phosphinic acid ethyl ester

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| for 12h; Heating; | 92% |
-
-
96-17-3, 57456-98-1
2-Methylbutyraldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
78023-52-6
(E)-ethyl 4-methylhex-2-enoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 92% |
| With manganese In tetrahydrofuran for 3h; Reflux; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 92% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
24393-56-4
ethyl (E)-3-(4-methoxyphenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 92% |
| With manganese In tetrahydrofuran for 3h; Reflux; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 92% |
-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
15186-48-8
2,3-isopropylidene-glyceraldehyde

-
-
64520-58-7
ethyl (E)-3-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)acrylate

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 75℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 91% |
-
-
98-86-2
acetophenone

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
91767-65-6
2-chloro-3-hydroxy-3-phenyl-butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| bronze-coloured C8K; silver(I) acetate; zinc(II) chloride In tetrahydrofuran at -20℃; for 0.666667h; | 90% |
| With amalgamated magnesium; diethyl ether |
-
-
78-84-2
isobutyraldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
21806-21-3, 78058-54-5
ethyl 2-chloro-3-isopropyloxirane-2-carboxylate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In diethyl ether 0-5 deg C, 1 h; reflux, 3 h; | 90% |
| With base In diethyl ether Darzens condensation; | |
| With sodium ethanolate In diethyl ether; ethanol at 0℃; for 1h; |
-
-
104-55-2
3-phenyl-propenal

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
38447-06-2, 38448-85-0, 39806-16-1, 136265-12-8, 1552-95-0, 106925-11-5
(2E)-ethyl 5-phenylpenta-2,4-dienoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 90% |
-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
120522-10-3
2,3,4,5-di-O-isopropylidenealdehydo-L-xylose

-
-
1310802-46-0
ethyl (E)-2,3-dideoxy-4,5:6,7-di-O-isopropylidene-L-xylo-hept-2-enonate

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 75℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; Aliquat 336 In toluene for 8h; Ambient temperature; | 89% |
-
-
590-86-3
isovaleraldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
34993-63-0
ethyl (E)-5-methylhex-2-enoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 89% |
| With manganese In tetrahydrofuran for 3h; Reflux; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 89% |
-
-
120-92-3
cyclopentanone

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
114091-86-0
chloro-(1-hydroxy-cyclohexyl)-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| bronze-coloured C8K; silver(I) acetate; zinc(II) chloride In tetrahydrofuran at 0℃; for 1h; | 88% |
-
-
112-45-8
10-Undecenal

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
148118-58-5
ethyl (E)-trideca-2,12-dienoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 88% |
| With manganese In tetrahydrofuran for 3h; Reflux; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 88% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
24393-52-0
ethyl (E)-3-(4-chlorophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With manganese In tetrahydrofuran for 3h; Heating; | 88% |
| With manganese In tetrahydrofuran for 3h; Reflux; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 88% |
-
-
23558-05-6
3-O-benzyl-1,2-O-isopropylidene-1,5-D-xylo-dialdofuranose

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
108788-49-4
ethyl (E)-[3-O-benzyl-5,6-dideoxy-1,2-O-isopropylidene-α-D-gluco]-heptofuran-5-en-uronate

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 75℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In diethyl ether 0-5 deg C, 1 h; reflux, 3 h; | 86% |
| With sodium ethanolate 1.) ether, 30 min, 2.) ether, reflux, 3 h; Multistep reaction; | |
| With base In diethyl ether Darzens condensation; |
-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
125951-38-4
ethyl 2-chloro-2-deuterioacetate

| Conditions | Yield |
|---|---|
| With tributyltin deuteride for 24h; Ambient temperature; | 86% |
-
-
52784-38-0
2-acetyl-2-cyclohexen-1-one

-
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 3h; | 86% |
-
-
536-74-3
phenylacetylene

-
-
535-15-9
ethyl 1,1-dichloroacetate

-
-
52293-00-2
1,1-dichloro-4-phenylbut-3-yn-2-one

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: ethyl 1,1-dichloroacetate With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at 20℃; for 2h; Further stages.; | 85% |
Acetic acid,2,2-dichloro-, ethyl ester Specification
The Acetic acid,2,2-dichloro-, ethyl ester with CAS registry number of 535-15-9 is also known as Acetic acid, dichloro-, ethyl ester. The IUPAC name is Ethyl 2,2-dichloroacetate. It belongs to product categories of C2 to C5; Carbonyl Compounds; Esters. Its EINECS registry number is 208-611-6. In addition, the formula is C4H6Cl2O2 and the molecular weight is 157. What's more, This chemical can be used as solvent and medicine synthesis intermediates.
Physical properties about Acetic acid,2,2-dichloro-, ethyl ester are: (1)ACD/LogP: 1.52; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.52; (4)ACD/LogD (pH 7.4): 1.52; (5)ACD/BCF (pH 5.5): 8.45; (6)ACD/BCF (pH 7.4): 8.45; (7)ACD/KOC (pH 5.5): 160.34; (8)ACD/KOC (pH 7.4): 160.34; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 26.3Å2; (13)Index of Refraction: 1.441; (14)Molar Refractivity: 32.01 cm3; (15)Molar Volume: 121.1 cm3; (16)Polarizability: 12.69×10-24cm3; (17)Surface Tension: 32.9 dyne/cm; (18)Density: 1.295 g/cm3; (19)Flash Point: 55.6 °C; (20)Enthalpy of Vaporization: 38.8 kJ/mol; (21)Boiling Point: 151.1 °C at 760 mmHg; (22)Vapour Pressure: 3.72 mmHg at 25 °C.
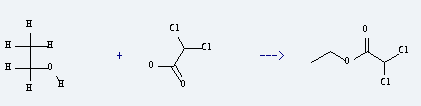
Preparation of Acetic acid,2,2-dichloro-, ethyl ester: it is prepared by reaction of dichloroacetic acid with ethanol. The reaction needs reagents P2O5, CuSO4, Na2SO4 and other condition of heating for 2 hours. The yield is about 40%.
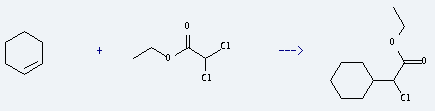
Uses of Acetic acid,2,2-dichloro-, ethyl ester: it is used to produce a-Chlor-cyclohexyl-essigsaeureaethylester by reaction with Cyclohexene. The reaction occurs with reagents Na2S2O4, NaHCO3 and solvent dimethylsulfoxide at 75 °C for 7 hours. The yield is about 65%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes and respiratory system. This chemical is Harmful by inhalation and in contact with skin. During using it, wear suitable protective clothing and do not breathe gas/fumes/vapour/spray. In case of contact with eyes, rinse immediately with plenty of water and seek medicl advice.
You can still convert the following datas into molecular structure:
1. SMILES: ClC(Cl)C(=O)OCC
2. InChI: InChI=1/C4H6Cl2O2/c1-2-8-4(7)3(5)6/h3H,2H2,1H3
3. InChIKey: IWYBVQLPTCMVFO-UHFFFAOYAQ
4. Std. InChI: InChI=1S/C4H6Cl2O2/c1-2-8-4(7)3(5)6/h3H,2H2,1H3
5. Std. InChIKey: IWYBVQLPTCMVFO-UHFFFAOYSA-N
Related Products
- Acetic (2H)acid
- Acetic acid 2-methylcyclohexyl ester
- Acetic acid 3-methoxy-3-methylbutyl ester
- Acetic acid acetoxy-(4-chlorosulfonylphenyl)methyl ester
- Acetic acid ethenyl ester, polymer with ethene and sodium ethenesulfonate
- Acetic acid ethenyl ester, polymer with ethenol
- Acetic acid glacial
- Acetic acid methylnitrosaminomethyl ester
- Acetic acid myrcenyl ester
- Acetic acid tetrahydro-2H-thiopyran-2-yl ester
- 5351-63-3
- 5351-69-9
- 535-17-1
- 53517-14-9
- 53518-14-2
- 53518-15-3
- 53518-18-6
- 53518-91-5
- 5351-91-7
- 53520-66-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View