-
Name
Allylacetic acid
- EINECS 228-028-0
- CAS No. 591-80-0
- Article Data107
- CAS DataBase
- Density 0.993 g/cm3
- Solubility Soluble in ethanol and diethyl ether, slightly soluble in water
- Melting Point 54-57 °C(lit.)
- Formula C5H8O2
- Boiling Point 188.499 °C at 760 mmHg
- Molecular Weight 100.117
- Flash Point 78.683 °C
- Transport Information UN 2922
- Appearance clear colorless to light yellow liquid
- Safety 26-36/37/39-45
- Risk Codes 22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Pent-4-enoic acid;NSC 9000;delta 4-Pentenoic acid;
- PSA 37.30000
- LogP 1.03720
Synthetic route

| Conditions | Yield |
|---|---|
| With poly-4-vinylpyridine In N,N-dimethyl-formamide for 0.0666667h; microwave irradiation; | 96% |
| Stage #1: allylmalonic acid In N,N-dimethyl-formamide at 140℃; Stage #2: With hydrogenchloride In N,N-dimethyl-formamide pH=4; | 85% |
| In N,N-dimethyl-formamide at 140℃; Inductive heating; | 76% |
-
-
90711-60-7
prop-2-en-1-yl 2-iodoacetate

-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| Stage #1: prop-2-en-1-yl 2-iodoacetate With triethyl borane; oxygen In hexane; dichloromethane at 20℃; for 2h; Stage #2: With zinc In tetrahydrofuran for 4h; Heating; | 85% |

-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide; tetrabutylammomium bromide In tetrahydrofuran; water for 12h; Heating; | 84% |
-
-
64096-43-1
β-(trimethylsilyl)cyclopentanone

-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In water; acetonitrile at 60℃; for 1.5h; Ring cleavage; | 82% |
-
-
69962-08-9
(Z)-5-(Ethylthio)-3-pentenoic Acid

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
33698-87-2
(Z)-pent-3-enoic acid

| Conditions | Yield |
|---|---|
| With ammonia; lithium for 1h; | A 8% B 70% |
-
-
2067-33-6
5-bromopentanoic acid

-
-
107-19-7
propargyl alcohol

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
103675-09-8
5-Hydroxy-pentanoic acid prop-2-ynyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane 1) -80 deg C, 2) room temp., 18 h; | A 5% B 70% |


-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With zinc In tetrahydrofuran | 63% |

| Conditions | Yield |
|---|---|
| With chromic acid; acetone | 59% |
| With chromium(VI) oxide; sulfuric acid In acetone |

| Conditions | Yield |
|---|---|
| copper(l) chloride at 0℃; for 0.25h; | 59% |
-
-
142920-94-3
2-Benzoyl-3-oxo-hept-6-enoic acid methyl ester

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
36568-12-4
methyl 3,5-dioxo-5-phenylpentanoate

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; water; ammonium chloride | A 43% B 56% |
-
-
25055-86-1
3-(3-methyl-3H-diazirine-3-yl)propionic acid

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
33698-87-2
(Z)-pent-3-enoic acid

-
C
-
1617-32-9
(E)-3-pentenoic acid

-
D
-
108-29-2
5-methyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| In xylene at 80℃; Kinetics; Product distribution; Mechanism; Ea; other temperatures (95-125 deg C); other solvent; | A 18.7% B 20% C 56% D 5.3% |
| In pentane Product distribution; Mechanism; Irradiation; other solvent; |

-
-
75-77-4
chloro-trimethyl-silane

-
-
6089-09-4
4-pentynoic acid

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
128545-15-3
5-trimethylsilyl-pent-5-yn-1-oic acid

| Conditions | Yield |
|---|---|
| With copper; zinc In acetonitrile at 120℃; for 5h; in a sealed tube; Yields of byproduct given; | A n/a B 52% |

-
-
129731-35-7
6,12,13-Trioxadispiro<4.1.4.2>tridecan

-
A
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
B
-
591-80-0
pent-4-enoic acid

-
C
-
2561-88-8
1-Oxacycloundecan-2,10-dion

-
D
-
120-92-3
cyclopentanone

| Conditions | Yield |
|---|---|
| In pentane at -20℃; for 8h; Irradiation; | A 16% B 7% C 45% D 14% |


| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In toluene at 90℃; for 48h; | 42% |
| With bis(tri-n-butyltin)oxide In toluene for 48h; Heating; | 42% |
| (alkaline hydrolysis); | |
| With potassium hydroxide Yield given; |
-
-
109-99-9
tetrahydrofuran

-
-
84210-61-7
perpentene-4 oate de tertiobutyle

-
A
-
1004-29-1
2-butanyltetrahydrofuran

-
B
-
1592-33-2
octahydro-2,2′-bifuran

-
C
-
1927-59-9
2-tert-butoxytetrahydrofuran

-
D
-
591-80-0
pent-4-enoic acid

-
E
-
32933-12-3
acide (tetrahydrofuryl-2)-5 pentanoique

-
F
-
93676-72-3
(tetrahydrofuryl-2)-5 pentanolide-4

| Conditions | Yield |
|---|---|
| at 110℃; for 8h; Product distribution; Mechanism; other temperatures, other times, also with various ethers, other products; | A 6% B 7% C 4% D 6% E 17% F 42% |

-
-
109-99-9
tetrahydrofuran

-
-
84210-61-7
perpentene-4 oate de tertiobutyle

-
A
-
1592-33-2
octahydro-2,2′-bifuran

-
B
-
591-80-0
pent-4-enoic acid

-
C
-
32933-12-3
acide (tetrahydrofuryl-2)-5 pentanoique

-
D
-
93676-72-3
(tetrahydrofuryl-2)-5 pentanolide-4

| Conditions | Yield |
|---|---|
| at 110℃; for 8h; Further byproducts given; | A 7% B 6% C 17% D 42% |


-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With zinc In tetrahydrofuran | 35% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-butene With magnesium In tetrahydrofuran at 20 - 70℃; Stage #2: carbon dioxide In tetrahydrofuran at -50 - -40℃; Stage #3: With hydrogenchloride In tetrahydrofuran; water | 35% |

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); 2.9-dimethyl-1,10-phenanthroline; silver fluoride In N,N-dimethyl acetamide at 20℃; under 5171.62 Torr; for 24h; | A 7% B 21% |


| Conditions | Yield |
|---|---|
| Stage #1: cyclopentanone With dihydrogen peroxide Stage #2: With copper(II) sulfate; iron(II) sulfate | 20% |
| With dihydrogen peroxide; copper(II) sulfate; iron(II) sulfate 1.) MeOH, 20 - 25 deg C, 1 h, 2.) 18- 20 deg C; Yield given. Multistep reaction; |
-
-
2067-33-6
5-bromopentanoic acid

-
-
927-74-2
1-butyn-4-ol

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
103675-14-5
9-hydroxy-non-6-ynoic acid

-
C
-
103675-17-8
5-Hydroxy-pentanoic acid but-3-ynyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane 1) -110 deg C, 2) room temp., 18 h; Yield given; | A 10% B 5% C n/a |


| Conditions | Yield |
|---|---|
| With manganese; (1,2-dimethoxyethane)dichloronickel(II); 2.9-dimethyl-1,10-phenanthroline In N,N-dimethyl acetamide at 20℃; under 5171.62 Torr; for 14h; | 8% |
-
-
2067-33-6
5-bromopentanoic acid

-
-
107-19-7
propargyl alcohol

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
103675-11-2
8-hydroxyoct-6-ynoic acid

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane 1) -30 deg C, 2) room temp., 18 h; Yield given; | A 5% B n/a |
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide Product distribution; 1) -30 deg C, 2) room temp., 18 h; other temperature; |

-
-
2067-33-6
5-bromopentanoic acid

-
-
927-74-2
1-butyn-4-ol

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
103675-14-5
9-hydroxy-non-6-ynoic acid

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane 1) -30 deg C, 2) room temp., 18 h; Yield given; | A 5% B n/a |
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide Product distribution; 1) -30 deg C, 2) room temp., 18 h; other temperature; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite | |
| With hydrogenchloride; sodium nitrite |
-
-
38300-67-3
1,2,4-Tribromobutane

-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; magnesium und man laesst auf das Reaktionsprodukt CO2 einwirken; |
-
-
2583-25-7
allylmalonic acid

-
A
-
591-80-0
pent-4-enoic acid

-
B
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

| Conditions | Yield |
|---|---|
| at 180℃; |

| Conditions | Yield |
|---|---|
| With sodium ethanolate at 150 - 160℃; und nachfolgendes Verseifen des Aethylesters durch Erwaermen mit alkoholischer Kalilauge; | |
| With potassium hydroxide; water |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium phenylselenide In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| (i) nBuLi, (ii) /BRN= 635704/, (iii) aq. HCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With iodine; sodium hydrogencarbonate; potassium iodide for 36h; Ambient temperature; | 100% |
| With iodine; sodium hydrogencarbonate; potassium iodide In water for 12h; Ambient temperature; | 98% |
| With oxone; potassium iodide In acetonitrile for 0.5h; | 97% |
-
-
591-80-0
pent-4-enoic acid

-
-
1666-13-3
diphenyl diselenide

-
-
65234-93-7
5-phenylselanylmethyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With copper(I) trifluoromethanesulfonate benzene; calcium carbonate In dichloromethane at 30℃; for 8h; | 100% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile at 30℃; | 95% |
| With carbon tetrabromide In dichloromethane for 4h; Irradiation; | 95% |
-
-
591-80-0
pent-4-enoic acid

-
-
39928-72-8
5-chloromethyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; magnesium chloride In dichloromethane; water at 20℃; for 0.666667h; | 100% |
| Stage #1: pent-4-enoic acid With oxalyl dichloride; 1,1'-sulfinylbisbenzene In dichloromethane at -78 - 20℃; for 1h; Inert atmosphere; Stage #2: With potassium carbonate In acetonitrile at 20℃; for 2h; Reagent/catalyst; Inert atmosphere; | 88% |
| With [bis(acetoxy)iodo]benzene; lithium chloride In methanol at 20℃; for 1h; | 73% |
-
-
591-80-0
pent-4-enoic acid

-
-
211745-86-7
pent-4-enoic acid (benzylcarbamoyl-methyl)-[(4-methoxy-benzylcarbamoyl)-methyl]-amide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; Acylation; | 100% |
-
-
591-80-0
pent-4-enoic acid

-
-
591-19-5
3-bromoaniline

-
-
263546-11-8
pent-4-enoic acid (3-bromo-phenyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane | 100% |
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide Acylation; |
-
-
591-80-0
pent-4-enoic acid

-
-
1329167-98-7
tert-butyl 1-(6-(2-amino-4-nitrophenyl)-3-chloropyridazin-4-yl)but-3-enylcarbamate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1.5h; | 100% |
-
-
2403-54-5
2-(4-chlorophenyl)-1,3-dioxolane

-
-
591-80-0
pent-4-enoic acid

-
-
1401468-38-9
5-(4-(1,3-dioxolan-2-yl)benzyl)dihydrofuran-2(3H)-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 2,2,2-trifluoroethanol for 6h; Inert atmosphere; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: pent-4-enoic acid With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 0.5h; Stage #2: 3-methyl-butane-1,3-diol In dichloromethane at 20℃; | 100% |
-
-
591-80-0
pent-4-enoic acid

-
-
41879-39-4
O-(tert-butyldimethylsilanyl)hydroxylamine

| Conditions | Yield |
|---|---|
| Stage #1: pent-4-enoic acid With 4-methyl-morpholine; chloroformic acid ethyl ester In diethyl ether at 0℃; for 1.5h; Inert atmosphere; Stage #2: O-(tert-butyldimethylsilanyl)hydroxylamine In diethyl ether at 0 - 20℃; for 4.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; diisopropyl-carbodiimide In dichloromethane at 0 - 20℃; for 1.16667h; | 100% |
-
-
591-80-0
pent-4-enoic acid

-
-
10374-51-3
5-hydroxymethyl-4,5-dihydrofuranone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dihydrogen peroxide In methanol; formic acid | 99% |
| With formic acid; dihydrogen peroxide at 50℃; for 2.25h; | 97% |
| With dihydrogen peroxide In tert-butyl alcohol | 88% |
-
-
591-80-0
pent-4-enoic acid

-
-
39716-58-0
pent-4-enoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane at 20℃; for 4h; Substitution; | 99% |
| With thionyl chloride; N,N-dimethyl-formamide for 16h; Inert atmosphere; Reflux; Schlenk technique; | 99% |
| With thionyl chloride at 70℃; for 3h; | 93% |
-
-
591-80-0
pent-4-enoic acid

-
-
3240-34-4
[bis(acetoxy)iodo]benzene

-
-
5904-80-3, 79580-69-1, 112607-21-3, 112709-12-3
(5-oxotetrahydrofuran-2-yl)methyl acetate

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In acetic acid at 80℃; for 17h; | 99% |
| With tetrafluoroboric acid In acetic acid for 1h; Ambient temperature; | 73% |
-
-
591-80-0
pent-4-enoic acid

-
-
32730-32-8
5-bromomethyl-γ-butyrolactone

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane at 20℃; for 4.5h; Darkness; Molecular sieve; | 99% |
| Stage #1: pent-4-enoic acid With dmap; bis(4-methoxyphenyl)selenide In chloroform at 0℃; for 0.25h; Stage #2: With N-Bromosuccinimide; sodium hydrogencarbonate In chloroform at 0℃; for 16h; Darkness; Inert atmosphere; regioselective reaction; | 98% |
| With Oxone; potassium bromide In nitromethane at -10℃; for 23h; Green chemistry; | 97% |
-
-
591-80-0
pent-4-enoic acid

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
95091-90-0
N-methoxy-N-methyl-4-pentenamide

| Conditions | Yield |
|---|---|
| Stage #1: pent-4-enoic acid With 1,1'-carbonyldiimidazole In dichloromethane at 0℃; for 0.5h; Stage #2: N,O-dimethylhydroxylamine*hydrochloride In dichloromethane at 0 - 20℃; | 99% |
| Stage #1: pent-4-enoic acid With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 1h; Stage #2: N,O-dimethylhydroxylamine*hydrochloride In dichloromethane at 20℃; for 16h; | 84% |
| Stage #1: pent-4-enoic acid With thionyl chloride at 60℃; for 5h; Stage #2: N,O-dimethylhydroxylamine*hydrochloride With triethylamine In dichloromethane at 0℃; for 0.5h; | 83% |
-
-
591-80-0
pent-4-enoic acid

-
-
171562-32-6
N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl)-4-methylbenzenesulfonamide

-
-
365449-54-3
(1S,2R)-1-(tosylamino)-2,3-dihydro-1H-inden-2-yl pent-4-enoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 23℃; | 99% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 3h; | 99% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 23℃; for 4h; | 4.31 g |

| Conditions | Yield |
|---|---|
| In benzene Heating; | 99% |
-
-
591-80-0
pent-4-enoic acid

-
-
99532-86-2
N,2-[(1,1-dimethylethoxy)carbonyl]-L-lysine methyl ester hydrochloride

-
-
787635-38-5
2-tert-butoxycarbonylamino-6-pent-4-enoylamino-hexanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 99% |
-
-
591-80-0
pent-4-enoic acid

-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
734534-08-8
N-[2-(3,4-dimethoxyphenyl)ethyl]-4-pentenamide

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 1.5h; under nitrogen atmosphere; | 99% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine | 95% |
-
-
473742-52-8
methyl 5-[2-(1-methyl-1H-indol-3-yl)ethyl]amino-1,3,4-thiadiazole-2-carboxylate

-
-
591-80-0
pent-4-enoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 25℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; Cooling with ice; | 99% |
-
-
591-80-0
pent-4-enoic acid

-
-
566203-48-3
(-)-1-[3-(4-methoxybenzyloxy)-1-penten-4-yloxycarbonyl]prop-2-yl 5-hydroxy-4-[(2-methoxyethoxy)methoxy]-2-hexenoate

-
-
1174412-33-9
C32H46O11

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; Inert atmosphere; | 99% |
-
-
591-80-0
pent-4-enoic acid

-
-
1357059-20-1
(E)-N-allyl-N-(3-hydroxybut-1-enyl)-4-methylbenzenesulfonamide

-
-
1357059-23-4
(E)-4-(N-allyl-4-methylphenylsulfonamido)but-3-en-2-yl pent-4-enoate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 0 - 20℃; for 15h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: pent-4-enoic acid With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 0.5h; Stage #2: 2,2-Dimethyl-1,3-diaminopropane In dichloromethane at 20℃; | 99% |
-
-
591-80-0
pent-4-enoic acid

-
-
4291-69-4, 6386-24-9, 6564-72-3, 59531-24-7, 69257-52-9, 78184-89-1, 78609-16-2, 78609-17-3, 78609-18-4, 96553-53-6, 104111-61-7, 131347-08-5, 4132-28-9
2,3,4,6-Tetra-O-benzyl-D-glucopyranose


| Conditions | Yield |
|---|---|
| With (R)-(+)-2-phenyl-2,3-dihydrobenzo[d]imidazo[2,1-b]thiazole; 2,2-dimethylpropanoic anhydride; N-ethyl-N,N-diisopropylamine In chloroform at 20℃; for 24h; Inert atmosphere; diastereoselective reaction; | A 99% B n/a |

-
-
591-80-0
pent-4-enoic acid

-
-
1016-58-6
6-nitroveratryl alcohol

-
-
609355-48-8
4-pentenoic acid 4,5-dimethoxy-2-nitrobenzyl

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 0 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 130℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate; toluene-4-sulfonic acid In 1,2-dichloro-ethane for 16h; Sealed tube; Reflux; | 98% |
| With ZrOTf-BTC In octane at 120℃; for 18h; Sealed tube; Inert atmosphere; | 95% |
| silver trifluoromethanesulfonate; iron(III) chloride In 1,2-dichloro-ethane at 80℃; for 5h; | 93% |
-
-
591-80-0
pent-4-enoic acid

-
-
63521-92-6
4-pentenoic anhydride

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane Inert atmosphere; | 98% |
| With N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine In dichloromethane for 0.5h; Ambient temperature; | 84% |
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 2h; | |
| With 2,4,6-trimethyl-pyridine; carbon tetrabromide In N,N-dimethyl-formamide for 0.5h; UV-irradiation; | 75 %Spectr. |
-
-
591-80-0
pent-4-enoic acid

-
-
525600-76-4
(R)-[2-(hydroxymethyl)-6-methyl-4-oxo-4H-1,3-dioxin-2-yl]methyl acetate

-
-
525601-14-3
(S)-{2-[(acetoxy)methyl]-6-methyl-4-oxo-4H-1,3-dioxin-2-yl}methyl pent-4-enoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 3h; cooling; | 98% |
Allylacetic acid Consensus Reports
Reported in EPA TSCA Inventory.
Allylacetic acid Specification
The Allylacetic acid, with the CAS registry number 591-80-0, is also known as Pent-4-enoic acid. It belongs to the product categories of Pharmaceutical Intermediates; omega-Functional Alkanols, Carboxylic Acids, Amines & Halides; omega-Unsaturated Carboxylic Acids. Its EINECS number is 228-028-0. This chemical's molecular formula is C5H8O2 and molecular weight is 100.12. What's more, its systematic name is 4-Pentenoic acid. This chemical should be sealed and stored at the temperature of 2 - 8 °C. It is an inhibitor of fatty acid oxidation. It can be used in the manufacturing of flavor concentrates of all types, and it is also used in organic synthesis.
Physical properties of Allylacetic acid are: (1)ACD/LogP: 0.984; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.04; (4)ACD/LogD (pH 7.4): -1.76; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 9.28; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.439; (14)Molar Refractivity: 26.503 cm3; (15)Molar Volume: 100.789 cm3; (16)Polarizability: 10.507×10-24cm3; (17)Surface Tension: 33.17 dyne/cm; (18)Density: 0.993 g/cm3; (19)Flash Point: 78.683 °C; (20)Enthalpy of Vaporization: 46.82 kJ/mol; (21)Boiling Point: 188.499 °C at 760 mmHg; (22)Vapour Pressure: 0.27 mmHg at 25°C.
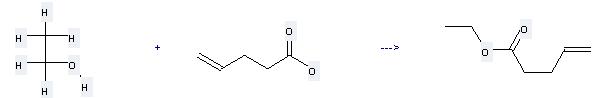
Preparation: this chemical can be prepared by pent-4-enoic acid ethyl ester at the temperature of 90 °C. This reaction will need reagent (Bu3Sn)2O and solvent toluene with the reaction time of 48 hours. The yield is about 42%.
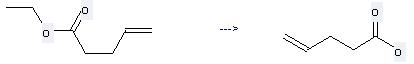
Uses of Allylacetic acid: it can be used to produce pent-4-enoic acid ethyl ester by heating. It will need reagent H2SO4 and solvent benzene with the reaction time of 15 hours. The yield is about 78%.
When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. It is harmful if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you should seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)CC\C=C
(2)Std. InChI: InChI=1S/C5H8O2/c1-2-3-4-5(6)7/h2H,1,3-4H2,(H,6,7)
(3)Std. InChIKey: HVAMZGADVCBITI-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 315mg/kg (315mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) ENDOCRINE: HYPOGLYCEMIA SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. |
| mouse | LD50 | oral | 610mg/kg (610mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
| mouse | LD50 | subcutaneous | 315mg/kg (315mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE ENDOCRINE: HYPOGLYCEMIA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. |
| rat | LD50 | oral | 470mg/kg (470mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
Related Products
- Allylacetic acid
- 591-81-1
- 591-82-2
- 59184-90-6
- 59186-41-3
- 591-87-7
- 59188-53-3
- 5918-93-4
- 59189-97-8
- 59191-99-0
- 59194-26-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View