-
Name
CYCLOPENTADIENYLMANGANESE TRICARBONYL
- EINECS 235-142-4
- CAS No. 12079-65-1
- Article Data64
- CAS DataBase
- Density
- Solubility Soluble in water (70 ppm (25C)
- Melting Point 72-76 °C(lit.)
- Formula C8H5MnO3
- Boiling Point 41.5oCat 760 mmHg
- Molecular Weight 204.064
- Flash Point
- Transport Information UN 2811 6.1/PG 2
- Appearance yellow crystals or crystalline chunks
- Safety 28-36/37-45
- Risk Codes 28
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms Cyclopentadienylmanganesetricarbonyl (6CI);Manganese, tricarbonyl-p-cyclopentadienyl- (8CI);Cyclopentadienyl-p-tricarbonylmanganese;Cyclopentadienyltricarbonylmanganese;Cymantrene;Manganesecyclopentadienyltricarbonyl;Tricarbonyl(h5-cyclopentadienyl)manganese;Tricarbonyl-p-cyclopentadienylmanganese;Tricarbonylcyclopentadienylmanganese;h5-Cyclopentadienylmanganese tricarbonyl;p-Cyclopentadienylmanganesetricarbonyl;
- PSA 0.00000
- LogP 0.39750
Synthetic route

| Conditions | Yield |
|---|---|
| in sealed tube at 200°C; | A 96% B 96% |

-
-
122-52-1
triethyl phosphite

-
A
-
12079-65-1
cymantrene

-
D
-
12276-93-6
[Mn(C5H5)(CO)2(P(OC2H5)3)]

-
E
-
64522-65-2
(C5H5)(CO)[P(OCH2CH3)3]MnCCHC6H5

| Conditions | Yield |
|---|---|
| In hexane at 20°C under Ar, 1 h; evapn., chromy. (Al2O3, hexane/ether, then ether), recrystn. (hexane/ether)., elem. anal.; | A n/a B 96% C n/a D n/a E <1 |


-
-
13292-87-0
dimethylsulfide borane complex

-
A
-
12079-65-1
cymantrene

-
B
-
40674-63-3
[(η5-cyclopentadienyl)Mn(CO)2SMe2]

-
C
-
2049-55-0
triphenylphosphine borane

| Conditions | Yield |
|---|---|
| In toluene Inert atmosphere; Schlenk technique; | A n/a B n/a C 95% |


-
-
116-17-6
triisopropyl phosphite

-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In hexane under Ar; addn. of P(O-i-Pr)3 hexane soln. to hexane soln. of complex, mixed 3h at 20°C; evapn., residue is dissolved in 1:1 hexane-diethyl ether mixt., chromd. (Al2O3), mixt. of CpMn(CO)3 and Cp(CO)2MnCCPhH, yellow band eluted with 1:1 hexane-methyl acetate, evapn. of solvent, oily residue treated with hexane/benzene, elem. anal.; | A n/a B 92% |

-
-
59699-77-3
cyclopentadienyl manganese tricarbonyl(1+)

-
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In dichloromethane Electrolysis; controlled potential electrolysis at 0.5 V, CH2Cl2/0.05 M N(C4H9)4B(C6F5)4; electrochemical IR-spectra; | 90% |
-
-
17685-52-8
triiron dodecarbonyl

-
-
109-79-5
1-butanethiol

-
-
121-44-8
triethylamine

-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran N2-atmosphere; stirring (room temp., 40 min), Mn-compd. addn., stirring (-90 to -70°C, 3 h); evapn. (vac., -50 to -40°C), chromy. (alumina, -25°C, petroleum ether, petroleum ether / CH2Cl2 = 20 : 1, petroleum ether / CH2Cl2/ Et2O = 10 : 1 : 1), evapn. (vac.), recrystn. (petroleum ether / CH2Cl 2, -80°C); elem. anal.; | A 3% B 85% C 6% D 4% |


| Conditions | Yield |
|---|---|
| In benzene Ar-atmosphere, dry solvent; Fe-compd. addn. to soln. of Mn,Pd-complex, stirring (3 h, 20°C), soln. filtration through Al2O3, vacuum evapn.; oily residue dissoln. in hexane-benzene, chromy. (Al2O3/hexane-benzene),eluate fractions evapn.; elem. anal.; | A n/a B 71% C 5% D 9% |

-
-
17685-52-8
triiron dodecarbonyl

-
-
645-96-5
Benzeneselenol

-
A
-
12079-65-1
cymantrene

-
B
-
349606-50-4
C5H5Mn(CO)2Fe(CO)3CC6H5SeC6H5

-
C
-
25987-99-9
(μ-PhSe)2Fe2(CO)6

| Conditions | Yield |
|---|---|
| With N(CH2CH3)3 In tetrahydrofuran (N2); addn. of selenium compd. and amine to a soln. of iron carbonyl in THF, stirring at room temp. for 10 min, cooling to -100°C, pouring to manganese complex, warming to -80°C, stirring for 7 h at -80to -50°C; evapn. at -50 to -40°C, column chromy (Al2O3, petroleum ether, petroleum ether/CH2Cl2 20:1, petroleum ether/CH2Cl2/Et2O 10:1:1), evapn., recrystn. (petroleum ether/CH2Cl2, -80°C); elem. anal.; | A 5% B 66% C 24% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With Pt(P(C6H5)3)4 In benzene a soln. of Mn-compd. (0.22 mmol) and Pt-compd. (0.20 mmol) in benzene was stirred under Ar at 20°C for 4 h;; evapn.; the residue was dissolved in hexane-benzene mixt. (2:1) and chromd. (alumina, hexane-benzene); removal of the solvent; residue was dissolved in ether; crystn. after cooling at -20°C;; | A n/a B 65% |

-
-
75-09-2
dichloromethane

-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In toluene (N2, Schlenk technique); addn. of molybdenum compd. to THF soln. of manganese compd., dissolving in toluene, stirring at room temp. for 15 min; evapn., extn. (CH2Cl2/petroleum ether (1:7)), chromy. (alumina, CH2Cl2/petroleum ether 1:7, then 1:1) at 253 K, evapn., elem. anal.; | A n/a B 65% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In pentane Ar atmosphere; stirring (20°C, 24 h); TLC (petroleum); | A n/a B 32% C 64% |

-
-
17685-52-8
triiron dodecarbonyl

-
-
37773-23-2
p-Selenocresol

-
A
-
12079-65-1
cymantrene

-
B
-
172367-46-3, 350039-26-8
(μ-p-MeC6H4Se)2Fe2(CO)6

-
C
-
349606-51-5
C5H5Mn(CO)2Fe(CO)3CC6H5SeC6H4CH3

| Conditions | Yield |
|---|---|
| With N(CH2CH3)3 In tetrahydrofuran (N2); addn. of selenium compd. and amine to a soln. of iron carbonyl in THF, stirring at room temp. for 10 min, cooling to -100°C, pouring to manganese complex, warming to -80°C, stirring for 7-8 h at -80 to -45°C; evapn. at -50 to -40°C, column chromy (Al2O3, petroleum ether, petroleum ether/CH2Cl2 20:1, petroleum ether/CH2Cl2/Et2O 10:1:1), evapn., recrystn. (petroleum ether/CH2Cl2, -80°C); elem. anal.; | A 6% B 25% C 63% |


-
-
63814-56-2
tetramethylammonium hydridoirontetracarbonyl

-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2); addn. of the Mn complex at -90°C to the Fe compd. in THF with vigorous stirring, further stirring (-90 to -45°C, 4 h); evapn. to dryness (high vacuum, -45 to -40°C), chromy. (neutral alumina; light petroleum, then light petroleum/CH2Cl2; -25°C), collection, solvent removal (vac.), recrystn. (light petr. or light petr./CH2Cl2 at -80°C); elem. anal.; | A 25% B 63% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In dichloromethane Ar atmosphere; stirring (20°C, 6 h); evapn. (vac.), extn. (CHCl3), chromy. (SiO2, petroleum ether, petroleumether/CHCl3 50:1, 5:1 and 2:1); elem anal.; | A n/a B 5% C 4.5% D 60% E 10% |


| Conditions | Yield |
|---|---|
| In benzene under Ar; stirred at 70°C for 30 min; filtration (0.5 cm Al2O3), concn. in vac., chromy. (Al2O3, hexane-benzene), elem. anal.; | A n/a B 53% C 28% D >1 |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2); bubbling CO gas through a soln. of the Mn-Fe complex in THF at -40to -10°C for 4 h; removal of solvent (vac.), chromy. (neutral alumina; light petroleum, then light petroleum/CH2Cl2), collection, removal of solvents (vac.), recrystn. (light petroleum or light petroleum/CH2Cl2; -80°C); elem. anal.; | A 31% B 46% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2); addn. of the Mn complex to the Fe complex salt in THF with stirring at -90°C, further stirring (-90 to -80°C, 1 h, -60°C, 3 h); solvent removal (vac., -40°C), chromy. (-25°C, neutral alumina; light petroleum/CH2Cl2, then light petroleum/CH2Cl2/Et2O); | A 26% B 44% |

-
-
26042-63-7
silver(I) hexafluorophosphate

-
-
136378-96-6
C5H5(CO)2MnSC6H4NO2

-
A
-
12079-65-1
cymantrene

-
B
-
136379-20-9
{C5H5(CO)2Mn}2SC6H4NO2(1+)*PF6(1-)*0.5CH2Cl2 = {C5H5(CO)2Mn}2SC6H4NO2PF6*0.5CH2Cl2

| Conditions | Yield |
|---|---|
| In toluene carried out with exclusion of air and moisture; 1 equiv of AgPF6 added to a stirred soln. of the Pd complex; mixt. stirred for further 20 min. at room temp.; volume reduced in vac., pentane added, filtered through silanized silica gel, elution with CH2Cl2, crystn. from CH2Cl2-Et2O (1:1) at -30°C; elem. anal.; | A n/a B 42% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; phenyllithium In diethyl ether byproducts: LiCl; Ar atmosphere; addn. of PhLi in ether to soln. of Mn-complex, stirring (-60°C, 30 min, at -20°C fo 30 min), acidification (HCl inether); sepn. of LiCl, filtration), evapn. (vac.), chrpmy. (5°C, ether/CH2Cl2 (1:4), ether); | A n/a B 40% |

-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2); addn. of the Mn complex to the Fe complex salt in THF with stirring at -90°C, further stirring (-90 to -80°C, 1 h), warming to -50°C over 3 h; evapn. to dryness (high vac., -40°C), chromy. (-25°C, neutral alumina; light petroleum/CH2Cl2, then light petroleum/CH2Cl2/Et2O); elem. anal.; | A 23% B 39% C 19% |


-
A
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With phenyllithium In diethyl ether byproducts: LiCl; addn. of 1 N soln. of C6H5Li in ether (4.06 mmol) to soln. of (C5H5)(OC)2Mn(CHCCH(CH3)OH) (4.06 mmol) in ether, stirring mixt. for 0.5 h at-60°C, 0.5 h at -20°C, acidifying with 1 N HCl in ether (dry solvents, Ar-atmosphere); filtering to remove LiCl, evapg. solvent in vac., chromy. of residue on SiO2-column (5°C), eluation with ether (elem. anal.); | A n/a B 35% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; acetone byproducts: Me3SiOH; Irradiation (UV/VIS); under Ar, soln. of Mn-complex was stirred for 4-5 h at -15 °C during irradn. with Hg-lamp., sulfinylimide was used in slight excess, soln. was warmed up to room temp. after 30 min, stirring for 2-3 h; soln. was evapd. in vac., subl., residue was dissolved in wet acetone, mixt. was stood for several days at room temp., elem. anal.; HNSO-complexwas not isolated, detected by spectr. methods; | 99% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; acetone byproducts: PhNH2; Irradiation (UV/VIS); under Ar, soln. of Mn-complex was stirred for 4-5 h at -15 °C during irradn. with Hg-lamp., sulfinylimide was used in slight excess, soln. was warmed up to room temp. after 30 min, stirring for 2-3 h; soln. was evapd. in vac., subl., residue was dissolved in wet acetone, mixt. was stood for several days at room temp., elem. anal.; | 99% |

-
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With CH3COCl; AlCl3 In dichloromethane byproducts: HCl; (Ar), to CH3COCl in CH2Cl2 added AlCl3 for 10 min, Mn-complex in CH2Cl2 added dropwise for 3 h; hydrolysed, HCl added, extracted, washed with H2O, dried over Na2SO4, filtered, solvent removed in vac.; | 96% |

| Conditions | Yield |
|---|---|
| palladium(II) oxide In toluene byproducts: CO; (Ar); soln. of Mn complex and PdO was heated to reflux and the isonitrilederiv. was added, refluxing was continued for 6 h, react. was monitored by TLC; chromy. (Kieselgel, CH2Cl2/hexane); recrystn. from CH2Cl2/hexane; elem. anal.; | 95% |
-
-
12079-65-1
cymantrene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| Stage #1: cymantrene With n-butyllithium In tetrahydrofuran; hexane at -78℃; Inert atmosphere; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran; hexane at -78 - 20℃; for 2h; | 94.5% |
| With n-butyllithium; ammonium chloride In tetrahydrofuran; Petroleum ether byproducts: (CH3)2NH; Ar; soln. of (OC)3MnC5H4Li, prepd. at -50°C from soln. of (OC)3MnC5H5 and soln. of n-butyl lithium in THF, cooled to -70°C, addedin 2-5 min to DMF; mixt. stirred (1-1.5 h, -10 - -5°C); satd. soln. of NH4Cl in H2O added; aq. layer washed (ether); org. extract dried (MgSO4); solvent distilled off; residue chromd. (SiO2, 1/2 petroleum ether-benzene); | 75% |
| With n-C4H9Li; NH4Cl In N,N-dimethyl-formamide (Ar); treatment soln. of cymantrene/THF with n-BuLi/C6H14 at -50°C for 15 min, cooling, transferring into Schlenk tube with DMF; stirringat 0°C for 90 min, addn. of soln. of NH4Cl; Bull. SSSR Acad. of Sci., Div. of Chemistry 29 (1990)2387; | 72% |
-
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With THF In tetrahydrofuran byproducts: CO; Irradiation (UV/VIS); irradn. until end of gas-evolution, 24 h stirred at 25°C, addn. of further C5H5Mn(CO)2(THF), stirred 24 h at 25°C.; recrystn. from THF-hexane; elem. anal.; | 94% |
-
-
12079-65-1
cymantrene

-
-
14580-91-7, 29218-61-9
trimethyl(methylene)phosphorane

| Conditions | Yield |
|---|---|
| In pentane stirred at 25°C for 20 min; cooling at 0°C, ppt. washed with ice-cold pentane, dried at 25°C, elem. anal.; | 93% |
-
-
75-79-6
Methyltrichlorosilane

-
-
12079-65-1
cymantrene

-
-
114835-71-1
tris(η5-cyclopentadienyl(tricarbonyl)manganese)methylsilane

| Conditions | Yield |
|---|---|
| With s-BuLi In tetrahydrofuran; cyclohexane To a soln. of Fe-compd. in THF a soln. of s-BuLi is added at -78°C, mixt. is stirred for 20 min. CH3SiCl3 is added dropwise via a syringe at -78°C, mixt. is allowed to warm to 20°C (N2).; Solvent is removed in vac., residue is chromd on alumina III, elem. anal.; | 93% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: HBr; under N2; cymantrene dissolved in neat BBr3; refluxed for 44 h; HBr condensed into cold trap (liq. N2); react. mixt. cooled to room temp.; filtered; filtrate evapd. slowly in vac.; detd. by X-ray powder diffraction; | 93% |
-
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| With n-butyl lithium; copper(II) chloride In diethyl ether; hexane ether soln. of CpMn(CO)3 was treated with hexane soln. of n-BuLi at -70°C in anaerobic conditions, stirred for 1 h at this temp., anhyd. CuCl2 was added, suspn. was stirred for 3 h at -60°C and brought to room temp. within 5 h; hydrolyzed (H2O), ether phase dried, evapd., residue was sublimed at 110°C under high vac.; elem. anal.; | 92% |
-
-
16567-18-3
8-bromoquinoline

-
-
12079-65-1
cymantrene

-
-
342813-38-1
tricarbonyl[η5-(8-quinolyl)cyclopentadienyl]manganese(I)

| Conditions | Yield |
|---|---|
| With n-butyllithium; ZnCl2; bis(triphenylphosphine)palladium(0) In tetrahydrofuran byproducts: LiCl; 1.) n-BuLi in hexane (-78°C), stirred for 1 h; 2.) ZnCl2 in THF, stirred for 1 h; 3.) Pd(PPh3)2, 8-bromoquinoline were added dropwise, the soln. was allowed to warm to room temp., stirred for 5 d (Ar, protection against light); aq. NaOH was added, stirred for 1.5 h, the organic layer was sepd., extd. with THF, dried (MgSO4), filtered, evapd., chromy. on Al2O3/5% H2O with toluene, elem. anal.; | 92% |
-
-
12079-65-1
cymantrene

-
-
79-03-8
propionyl chloride

-
-
62010-78-0
(Propionylcyclopentadienyl)tricarbonylmanganese

| Conditions | Yield |
|---|---|
| aluminium trichloride In carbon disulfide (Ar); Schlenk tube; AlCl3 was added to soln. of cymantrene and propionylchloride in CS2; after 1.5 h CS2 was removed in vac.; water was added at 0°C; extd. (Et2O); dried (MgSO4); soln. concd.; pentane added; recrystd. (Et2O/pentane); elem. anal.; | 92% |
| With AlCl3 In carbon disulfide (Ar); addn. of AlCl3 to a soln. of manganese complex in CS2, addn. of a soln. of propionyl chloride in CS2, stirring for 1.5 h; evapn., addn. of water at 0°C, extn. with Et2O, drying (MgSO4), concn., addn. of pentane, recrystn. (ether/ pentane); elem. anal.; | 92% |
| With aluminum (III) chloride In dichloromethane at 20℃; for 4h; Schlenk technique; | 71% |

-
-
12079-65-1
cymantrene

-
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| In diethyl ether; water Irradiation (UV/VIS); N2 atmosphere; addn. of org. comp. and aq. soln. of HPF6 to soln. of Mn-compd. in Et2O, purging with N2, stirring, irradiation without stirring (15-25°C, 15-30 min, 365 nm); 22% conversion; filtn., washing (ether), precipitaion from acetone/ether (0°C); elem. anal.; | 91% |

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
12079-65-1
cymantrene

-
-
14040-11-0
tungsten hexacarbonyl

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
407-25-0
trifluoroacetic anhydride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether; hexane treating 31 mmol (Mn(CO)3(C5H5)) in THF/ ether at -80°C with 32.0 mmol LiC4H9 in hexane; stirring for 1 h; addn. of 31.3 mmol (W(CO)6); stirring at -10°C for 1 h; cooling to -80°C; treating with (CF3CO)2O in THF; addn. of ligand;; warming to room temperature; evaporation under reduced pressure; extraction with dichloromethane; chromy. (CH2Cl2); concn. in vac.; diln. with petroleum; cooling to -80°C; pptn.; decantation; washing with petroleum; drying in vac.; elem. anal.;; | 91% |

-
-
75-21-8
oxirane

-
-
12079-65-1
cymantrene

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
215460-58-5
(η(5)-C5H4CH2CH2OSO2C6H4CH3)Mn(CO)3

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; pentane byproducts: LiCl; N2-atmosphere; addn. of equimolar amt. of BuLi (in pentane) to Mn-complex soln. (in THF) at -78°C, addn. of slight excess of ethylene oxide (after 3 h, -78°C), warming to 0°C (after 2 h), addn. of equimolar amt. of tosyl chloride; solvent removal (after 30 min, 25°C, vac.), extn. into CH2Cl2, filtration off of LiCl (Celite), evapn., chromy. (SiO2, CH2Cl2); elem. anal.; | 91% |


| Conditions | Yield |
|---|---|
| Stage #1: cymantrene With n-butyllithium In tetrahydrofuran; hexane at -80℃; for 1.5h; Schlenk technique; Inert atmosphere; Stage #2: oxirane In tetrahydrofuran; hexane at -80 - 25℃; Schlenk technique; Inert atmosphere; Stage #3: With water In tetrahydrofuran; hexane at 25℃; Schlenk technique; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| In further solvent(s) Irradiation (UV/VIS); cymantrene soln. in subcritical and supercritical ethylene (-40 - 50°C, 35-2600 bar), photolysis (10 h); not isolated; | 90% |
-
-
109-99-9
tetrahydrofuran

-
-
12079-65-1
cymantrene

-
-
83025-09-6
tetracarbonylbis(η5-cyclopentadienyl)-μ4-diarsenic-dimolybdenum (Mo-Mo)

-
A
-
111769-92-7
tetracarbonylbis(η5-cyclopentadienyl)μ4-diarsenic-bis{dicarbonyl(η5-cyclopentadienyl)manganese}dimolybdenum (Mo-Mo)

-
B
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); 4.90 mmol (C5H5)Mn(CO)3 in THF photolyzed for 90 min; soln. concd. and added to 4.28 mmol (C5H5)2Mo2(CO)4As2; soln. stirred for 6 h at room temp.; solvent concd.; chromy. (silica gel) with hexane yields (C5H5)Mn(CO)3, then with toluene/ether (1:1) (CpMo(CO)2)2(CpMn(CO)2As)2 which is crystd. from CH2Cl2/hexane at -18°C; elem. anal.; | A 90% B n/a |

-
-
12079-65-1
cymantrene

-
-
12267-07-1
(η5-C5H4(COPh))Re(CO)3

| Conditions | Yield |
|---|---|
| With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran; hexane cymantrenyllithium prepd. above -78°C from cymantrene, TMEDA, and n-BuLi; after 45 min, the Re complex is added; mixt. warmed to room temp. for 20 h; hydrolyzed; washed with distilled water; solvent removed under high vac.; chromy on silica with dichloromethane/hexane eluent; recrystd. from ethanol/water; elem. anal.; | 90% |
-
-
12079-65-1
cymantrene

-
-
593-75-9, 685498-28-6
methyl isocyanate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CO; Irradiation (UV/VIS); N2-atmosphere; equimolar amts.or excess MeNC, 100-W Hg lamp (18 h); evapn., chromy. (Al2O3), evapn., crystn. (-78°C, petroleum ether); elem. anal.; yield related to equimolar amts. of educts; | 90% |
| palladium(II) oxide In toluene byproducts: CO; (Ar); soln. of Mn complex and PdO was heated to reflux and the isonitrilederiv. was added, refluxing was continued for 24 h, react. was monitored by TLC; chromy. (Kieselgel, CH2Cl2/hexane); recrystn. from CH2Cl2/hexane; elem. anal.; | 5% |
-
-
12079-65-1
cymantrene

-
-
171562-97-3
[2,6-diphenylpyrylium](PF6)

-
-
341-02-6
trityl tetrafluoroborate

-
-
158840-67-6
[(4-cyclopentadienyl-2,6-diphenylpyrylium)Mn(CO)3](BF4)

| Conditions | Yield |
|---|---|
| With BuLi In tetrahydrofuran; diethyl ether; hexane N2-atmosphere; addn. of BuLi (hexane) to THF soln. of Mn-complex at -60°C, stirring for 1 h, addn. of pyrylium (THF:ether), warming to 0°C, addn. of aq. NH4Cl, extn. (ether), drying (MgSO4), chromy. (Al2O3, pentane), addn. of MeCN; addn. of Ph3CBF4, stirring for 30 min, addn. of ether, collection (filtration); elem. anal.; | 89% |


| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 20℃; for 5h; Friedel-Crafts Acylation; Schlenk technique; | 88% |
-
-
12079-65-1
cymantrene

-
-
1079-66-9
chloro-diphenylphosphine

-
-
62980-80-7
tricarbonyl(η5-diphenylphosphinocyclopentadienyl)manganese

| Conditions | Yield |
|---|---|
| With n-butyllithium In not given byproducts: LiCl; Complex was treated with n-BuLi at -70°C, PPh2Cl was added, the mixt. was kept at room temp. for 2 h;; aq. HCl was added with cooling, aq. NaOH was added to pH 8, extd. with ether or CH2Cl2, the organic layer was washed with water, evapd., crystd. from hexane or EtOH; elem. anal.;; | 87.4% |
-
-
12079-65-1
cymantrene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CO; Irradiation (UV/VIS); irradiating a soln. of Mn(CO)3(C2H5) until the CO evolution beeing finished (ca 2 - 3 h), adding the Rh complex, stirring (ca 24 h, room temp.); concg., chromy. (Al2O3, benzene), recrystn. (pentane); | 87% |
| In tetrahydrofuran byproducts: CO; Irradiation (UV/VIS); N2 atmosphere; photolysis of Mn-complex in THF (Hg-vapor lamp, 2-3 h), addn. of Rh-complex, stirring (room temp., 24 h); solvent removal, extraction (benzene), concn., chromy. (Al2O3, benzene/pentane 1:1), evapn. (vac.), crystn. (pentane, -78°C), collection (filtn.), washing (pentane), drying (vac.); elem. anal.; | 87% |
-
-
12079-65-1
cymantrene

-
-
63995-70-0
trisodium tris(3-sulfophenyl)phosphine

-
-
98511-67-2
tris(natrium-m-sulfonatophenyl)phosphanoxid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water byproducts: CO; Irradiation (UV/VIS); (N2 or Ar); irradiation of soln. of Mn-complex in THF (high pressure Hg-lamp, 90 min, 15°C), addn. to org. compd. in H2O, stirring (16 h); phase sepn., washing org. phase (H2O), aq. phase (n-pentane), evapn. (vac.), chromy. (Sephadex), chromy. (Fractogel, H2O/EtOH); elem. anal.; | A 87% B 25% |

CYCLOPENTADIENYLMANGANESE TRICARBONYL Chemical Properties
Product Name: Cyclopentadienylmanganese tricarbonyl (CAS NO.12079-65-1)
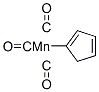
Molecular Formula: C8H5MnO3
Molecular Weight: 204.06g/mol
Mol File: 12079-65-1.mol
Einecs: 235-142-4
Appearance: Yellow crystals or crystalline chunks
Melting Point: 72-76 °C(lit.)
Boiling point: 41.5 °C at 760 mmHg
Sensitive: Air Sensitive
Enthalpy of Vaporization: 27.4 kJ/mol
Vapour Pressure: 418 mmHg at 25°C
H-Bond Donor: 0
H-Bond Acceptor: 4
Product Categories: ManganeseVapor Deposition Precursors; Catalysis and Inorganic Chemistry; Chemical Synthesis; Manganese; Precursors by Metal
CYCLOPENTADIENYLMANGANESE TRICARBONYL Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 710ug/kg (0.71mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#11285, | |
| mouse | LD50 | oral | 150mg/kg (150mg/kg) | BRAIN AND COVERINGS: "CHANGES IN CIRCULATION (HEMORRHAGE, THROMBOSIS, ETC.)" AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 28(4), Pg. 29, 1963. |
| rat | LCLo | inhalation | 120mg/m3/2H (120mg/m3) | VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION BLOOD: HEMORRHAGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Hygiene and Sanitation Vol. 30(4-6), Pg. 40, 1965. |
| rat | LD50 | intraperitoneal | 14mg/kg (14mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: CHRONIC PULMONARY EDEMA | Toxicology. Vol. 34, Pg. 341, 1985. |
| rat | LD50 | oral | 22mg/kg (22mg/kg) | Toxicology. Vol. 34, Pg. 341, 1985. |
CYCLOPENTADIENYLMANGANESE TRICARBONYL Consensus Reports
Reported in EPA TSCA Inventory. Manganese and its compounds are on the Community Right-To-Know List.
CYCLOPENTADIENYLMANGANESE TRICARBONYL Safety Profile
A poison by ingestion, inhalation, intraperitoneal, and intravenous routes. A mild narcotic which can damage kidneys. When heated to decomposition it emits acrid smoke and irritating fumes. See also MANGANESE COMPOUNDS and CARBON MONOXIDE.
Safety Information of Cyclopentadienylmanganese tricarbonyl (CAS NO.12079-65-1):
Hazard Codes: T+![]()
Risk Statements: 28
28: Very Toxic if swallowed
Safety Statements: 28-36/37-45
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
36: Wear suitable protective clothing
37: Wear suitable gloves
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
CYCLOPENTADIENYLMANGANESE TRICARBONYL Standards and Recommendations
OSHA PEL: TWA 0.1 mg(Mn)/m3 (skin)
ACGIH TLV: TWA 0.1 mg(Mn)/m3
CYCLOPENTADIENYLMANGANESE TRICARBONYL Specification
Cyclopentadienylmanganese tricarbonyl ,its CAS NO. is 12079-65-1,the synonyms is (Eta5-Cyclopentadienyl) manganese tricarbonyl ; Cyclopentadienyl-p-tricarbonylmanganese ; Cyklopentadientrikarbonylmanganium ; Cymantrene ; Manganese, cyclopentadienyltricarbonyl- ; Manganese, tricarbonyl-pi-cyclopentadienyl- ; manganese,cyclopentadienyltricarbonyl ; Manganese,tricarbonyl(η5-2,4-cyclopentadien-1-yl)- .
Related Products
- CYCLOPENTADIENYLMANGANESE TRICARBONYL
- 120796-97-6
- 120797-63-9
- 120800-52-4
- 120801-75-4
- 12080-32-9
- 1208-03-3
- 1208082-24-9
- 120-80-9
- 12081-16-2
- 1208-12-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View