-
Name
Iodoform
- EINECS 200-874-5
- CAS No. 75-47-8
- Article Data159
- CAS DataBase
- Density 3.864g/cm3
- Solubility insoluble in water, soluble in benzene,diethyl ether and acetone
- Melting Point 119-122 °C
- Formula CHI3
- Boiling Point 250.668 °C at 760 mmHg
- Molecular Weight 393.732
- Flash Point 128.988 °C
- Transport Information UN 1851
- Appearance yellow solid with a characteristic pungent and
- Safety 26-36/37/39-22
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Carbontriiodide;Dezinfekt V;NSC 26251;Triiodomethane;
- PSA 0.00000
- LogP 2.57500
Synthetic route

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In water; Petroleum ether at 0℃; for 12h; Reagent/catalyst; Solvent; | A 30% B 85% |


| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| at 100℃; |

| Conditions | Yield |
|---|---|
| With iodine | |
| With iodine in gleicher Weise entsteht es aus Aceton,Aldehyd,Milchsaeure usw.,ueberhaupt aus Koerpern,welche die Gruppen CH3.CO.C...oder CH3.CH(OH).C...enthalten; | |
| With water; iodine; potassium carbonate ueber mehrere Stufen; |

| Conditions | Yield |
|---|---|
| With calcium iodide at 100℃; |

| Conditions | Yield |
|---|---|
| beim Belichtung; |

| Conditions | Yield |
|---|---|
| With water; calcium oxide In methanol; water hydrolysis of CI4 in MeOH in the presence of CaO or sodium phenolate, no formation of CHI3;; | 0% |
| With water; potassium iodide In water formation of CHI3 on boiling CI4 with H2O in the presence of KI or dild. mineral acids;; | |
| With potassium cyanide | |
| With potassium hydroxide |


| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| With ammonium iodide; ammonia Electrolysis; |

| Conditions | Yield |
|---|---|
| With iodonitrogen |

-
A
-
64-18-6
formic acid

-
B
-
75-47-8
iodoform

-
C
-
624-74-8
diiodoacetylene

-
D
-
21701-42-8
piperidine hydroiodide

| Conditions | Yield |
|---|---|
| Destillation mit Wasserdampf; |


| Conditions | Yield |
|---|---|
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With alkaline solution; iodine | |
| With phosphate buffer; hypoiodous acid In water at 25℃; pH=6.8 - 8.6; Kinetics; Oxidation; |
-
-
594-68-3
triiodoacetic acid

-
-
64-19-7
acetic acid

-
A
-
75-47-8
iodoform

-
B
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate


| Conditions | Yield |
|---|---|
| With iodine; sodium carbonate |

| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| With alkaline iodine solution |
-
-
64-17-5
ethanol

-
-
79631-27-9
N-iodo-benzenesulfonamide; potassium salt

-
-
67-64-1
acetone

-
-
75-47-8
iodoform


| Conditions | Yield |
|---|---|
| With iodine In ethanol; water Rate constant; | |
| With sodium carbonate; potassium iodide Electrolysis; | |
| With sodium hydroxide; sodium hypochlorite; water; potassium iodide |

| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| With iodonitrogen |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 40℃; |
-
-
74-88-4
methyl iodide

-
A
-
75-11-6
diiodomethane

-
B
-
75-47-8
iodoform

-
C
-
34557-54-5
methane

-
D
-
74-84-0
ethane

| Conditions | Yield |
|---|---|
| bei der Einwirkung von UV-Licht der Wellenlaengen 253.7 nm; Prod.5.:Aethylen, Prod.6.:Jod; |


| Conditions | Yield |
|---|---|
| With OI(1-) In acetic acid at 25℃; Rate constant; Mechanism; | A n/a B n/a C 20 % Spectr. |



-
-
492-30-8, 7306-56-1, 19774-08-4, 36791-96-5, 36791-97-6, 53008-90-5, 53008-96-1, 53008-97-2, 86204-17-3, 86204-18-4, 93636-22-7
3,4-dihydroxy-5-hydroxymethyl-3-methyl-dihydro-furan-2-one

-
-
75-47-8
iodoform


| Conditions | Yield |
|---|---|
| Stage #1: methyl-malonic acid dimethylester With sodium hydride In tetrahydrofuran; mineral oil for 1.5h; Inert atmosphere; Reflux; Stage #2: iodoform In tetrahydrofuran; mineral oil at 50℃; for 16h; | 100% |
-
-
75-47-8
iodoform

-
-
609-08-5
Diethyl methylmalonate

-
-
311807-55-3
2-diiodomethyl-2-methyl-malonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl methylmalonate In diethyl ether for 0.5h; Stage #2: With sodium hydride In diethyl ether for 1.5h; Reflux; Stage #3: iodoform for 12h; Reflux; | 99% |
| With sodium hydride In diethyl ether Reflux; | 99% |
| Stage #1: Diethyl methylmalonate With sodium hydride In diethyl ether; mineral oil at 40℃; for 3h; Inert atmosphere; Stage #2: iodoform In diethyl ether; mineral oil for 24h; Inert atmosphere; Reflux; | 80% |
-
-
75-47-8
iodoform

-
-
329362-90-5
(2R,3R,4R,5S,E)-3,5-dimethoxy-2,4-dimethyl-7-phenylhept-6-enal

-
-
387821-97-8
1-((1E,3S,4R,5S,6S,7E)-8-iodo-3,5-dimethoxy-4,6-dimethylocta-1,7-dienyl)benzene

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Darkness; optical yield given as %de; diastereoselective reaction; | 99% |
| With chromium dichloride In tetrahydrofuran at 20℃; for 1h; | 39.6 mg |
-
-
75-47-8
iodoform

-
-
830-79-5
2,4,6-trimethoxybenzaldehyde

-
-
1374568-09-8
2,2-diiodo-1-(2,4,6-trimethoxyphenyl)ethanol

| Conditions | Yield |
|---|---|
| Stage #1: iodoform; 2,4,6-trimethoxybenzaldehyde With isopropylmagnesium chloride In tetrahydrofuran at -78 - 0℃; Inert atmosphere; Stage #2: With water; ammonium chloride In tetrahydrofuran | 99% |
-
-
75-47-8
iodoform

-
-
2283-11-6
hexaethylphosphoric triamide

-
-
40985-29-3
Tris(diethylamino)methylphosphonium-iodid

| Conditions | Yield |
|---|---|
| 98% |
-
-
75-47-8
iodoform

-
-
80885-30-9, 81028-03-7, 69152-88-1
(E)-4-(benzyloxy)-2-buten-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: iodoform; (E)-4-(benzyloxy)-2-buten-1-ol With iodine; diethylzinc In diethyl ether; dichloromethane at 0℃; Stage #2: With ammonium chloride In diethyl ether; dichloromethane for 0.0166667h; | 98% |
-
-
75-47-8
iodoform

-
-
53005-18-8
1,5-dimethoxy-pentan-3-one

-
-
938184-27-1
1-iodo-4-methoxy-2-(2-methoxyethyl)-but-1-ene

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 20℃; for 15h; Product distribution / selectivity; Kishi-Nozaki Coupling; | 98% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran; 1,4-dioxane at 20℃; for 12h; Takai olefination; | 96% |
-
-
75-47-8
iodoform

-
-
168556-11-4
[(C5H5)Mo(C9H7)I(CO)](1+)*BF4(1-)=[(C5H5)Mo(C9H7)I(CO)]BF4

| Conditions | Yield |
|---|---|
| In dichloromethane Irradiation (UV/VIS); Ar-atmosphere; irradn. (6 h); filtering, washing (ether), recrystn. (Me2CO / ether); elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran; 1,4-dioxane at 20℃; for 12h; Takai olefination; | 96% |
-
-
75-47-8
iodoform

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
265999-47-1
α-(diiodomethyl)-4-methoxybenzenemethanol

| Conditions | Yield |
|---|---|
| Stage #1: iodoform; 4-methoxy-benzaldehyde With isopropylmagnesium chloride In tetrahydrofuran at -78 - 0℃; Inert atmosphere; Stage #2: With water; ammonium chloride In tetrahydrofuran | 96% |
-
-
75-47-8
iodoform

-
-
30390-50-2, 65405-70-1, 21662-09-9
(Z)-4-decenal

-
-
148138-17-4
(1E,5Z)-1-iodoundeca-1,5-diene

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran | 95% |
| With chromium dichloride In tetrahydrofuran at 0℃; for 3h; | 94% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 0℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine | 95% |
-
-
75-47-8
iodoform

-
-
245730-36-3
(η(5);η(5)-fulvalene)W2(CO)4(P(C6H5)2CH3)2H2

-
-
214425-19-1, 214331-04-1, 214425-20-4, 214425-21-5
(η(5);η(5)-fulvalene)W2(CO)4(P(C6H5)2CH3)2I2

| Conditions | Yield |
|---|---|
| In toluene inert atmosphere; 2 equiv. of iodoform, stirring (room temp., overnight); concn. (reduced pressure), hexane addn., collection (filtration), washing (hexane), drying (vac.); isomer mixt. not sepd., detd. by (1)H-NMR spectroscopy; | 95% |
| In toluene inert atmosphere; excess of CHI3, stirring (room temp., overnight; pptn.); vol. reduction (vac.), hexane addn., collection (filtration), washing (hexane), drying (reduced pressure); isomer mixt. not sepd., detd. by (1)H- and (31)P-NMR spectroscopy; | 95% |
| In [(2)H6]acetone inert atmosphere; excess of CHI3, room temp.; not isolated, reaction followed by (1)H- and (31)P-NMR spectroscopy; |
-
-
75-47-8
iodoform

-
-
93292-50-3
(Z)-(R)-8-(tert-Butyl-diphenyl-silanyloxy)-9-oxo-non-5-enoic acid methyl ester

-
-
1252798-85-8
methyl (5Z,8R,9E)-8-[1-(tert-butyl)-1,1-diphenylsilyl]oxy-10-iodo-5,9-decadienoate

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 0 - 20℃; for 16h; Takai olefination; Inert atmosphere; Darkness; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: iodoform With sodium hexamethyldisilazane In tetrahydrofuran; diethyl ether at -78 - -20℃; for 1h; Inert atmosphere; Stage #2: 4-Methylbenzyl bromide In tetrahydrofuran; diethyl ether at -20℃; for 5h; Inert atmosphere; Stage #3: With potassium tert-butylate In tetrahydrofuran; diethyl ether at -20 - 20℃; for 12h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triphenylphosphine In tetrahydrofuran at -78℃; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In water stirring; washing, drying (vac., 24 h); elem. anal.; | 94.3% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran; 1,4-dioxane at 25℃; for 1h; | 94% |
| With chromium dichloride In tetrahydrofuran; 1,4-dioxane at 25℃; for 1.5h; | 94% |
-
-
75-47-8
iodoform

-
-
68972-96-3
(Z)-1,4-dibenzyloxy-2-butene

| Conditions | Yield |
|---|---|
| Stage #1: iodoform; (Z)-1,4-dibenzyloxy-2-butene With diethylzinc In dichloromethane Stage #2: With diclazuril; water-d2 In dichloromethane Further stages.; | 94% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triphenylphosphine | 94% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran; 1,4-dioxane at 20℃; for 5h; Takai-Utimoto olefination; Sealed tube; | 94% |
-
-
34841-06-0
3-bromo-4-methoxybenzylaldehyde

-
-
75-47-8
iodoform

-
-
1374568-21-4
1-(3-bromo-4-methoxyphenyl)-2,2-diiodoethanol

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-4-methoxybenzylaldehyde; iodoform With isopropylmagnesium chloride In tetrahydrofuran at -78 - 0℃; Inert atmosphere; Stage #2: With water; ammonium chloride In tetrahydrofuran | 94% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 20℃; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; Glovebox; | 94% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: naphthalene; (N2 or Ar), mixed at -78°C; warmed to room temp.; solvent removed; chromd. (alumina, CH2Cl2); recrystd. (toluene); | 93% |
-
-
15243-33-1
dodecacarbonyl-triangulo-triruthenium

-
-
75-47-8
iodoform

-
-
2519-10-0
1,2,3,4,5-pentaphenylcyclopentadiene

-
-
770729-72-1
(η5-pentaphenylcyclopentadienylato)Ru(CO)2I

| Conditions | Yield |
|---|---|
| In decane; toluene 160°C, excess CHI3; | 93% |


| Conditions | Yield |
|---|---|
| With chromium dichloride In tetrahydrofuran at 20℃; for 3h; Takai olefination; | 93% |
-
-
75-47-8
iodoform

-
-
100-52-7
benzaldehyde

-
A
-
4110-77-4
(E)-β-chlorostyrene

-
B
-
101349-79-5
2-phenylvinyl iodide

| Conditions | Yield |
|---|---|
| With chromium chloride; zinc In tetrahydrofuran at 20℃; for 4h; Takai reaction; | A 7% B 92% |

Iodoform Consensus Reports
Iodoform Standards and Recommendations
ACGIH TLV: TWA 0.6 ppm
Iodoform Specification
Iodoform, with the CAS registry number 75-47-8, is also named as Triiodomethane. It belongs to the product category of Organics. Its EINECS number is 200-874-5. This chemical's molecular formula is CHI3 and molecular weight is 393.73. What's more, its systematic name is Iodoform. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Mutation data; (3)Tumor data.This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, heat and fire. It is occasionally used as a disinfectant.
Physical properties of Iodoform are: (1)ACD/LogP: 3.118; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.12; (4)ACD/LogD (pH 7.4): 3.12; (5)ACD/BCF (pH 5.5): 138.00; (6)ACD/BCF (pH 7.4): 138.00; (7)ACD/KOC (pH 5.5): 1183.94; (8)ACD/KOC (pH 7.4): 1183.94; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Index of Refraction: 1.851; (13)Molar Refractivity: 45.549 cm3; (14)Molar Volume: 101.897 cm3; (15)Polarizability: 18.057×10-24cm3; (16)Surface Tension: 66.8 dyne/cm; (17)Density: 3.864 g/cm3; (18)Flash Point: 128.988 °C; (19)Enthalpy of Vaporization: 46.821 kJ/mol; (20)Boiling Point: 250.668 °C at 760 mmHg; (21)Vapour Pressure: 0.03 mmHg at 25°C.
Preparation of Iodoform: this chemical can be synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: (i) a methyl ketone: CH3COR, acetaldehyde, ethanol, and certain secondary alcohols (CH3CHROH, where R is an alkyl or aryl group).
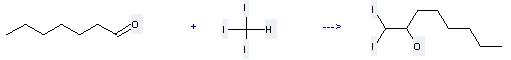
Uses of Iodoform: it can be used to produce 1,1-diiodo-octan-2-ol at the temperature of 0 °C. It will need reagents samarium iodide, 1 M HCl and solvent tetrahydrofuran with the reaction time of 45 min. The yield is about 60%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. You should not breathe dust. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: IC(I)I
(2)Std. InChI: InChI=1S/CHI3/c2-1(3)4/h1H
(3)Std. InChIKey: OKJPEAGHQZHRQV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | 10gm/kg (10000mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: TREMOR | Zeitschrift fuer Experimentelle Pathologie und Therapie. Vol. 1, Pg. 446, 1905. |
| frog | LDLo | unreported | 606mg/kg (606mg/kg) | Zeitschrift fuer Experimentelle Pathologie und Therapie. Vol. 1, Pg. 446, 1905. | |
| guinea pig | LD50 | oral | 487mg/kg (487mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Zdravookhranenie Turkmenistana. Public Health of Turkmenistan. Vol. 27(5), Pg. 9, 1983. |
| mammal (species unspecified) | LDLo | oral | 1gm/kg (1000mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| mammal (species unspecified) | LDLo | subcutaneous | 1500mg/kg (1500mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| mouse | LD50 | oral | 470mg/kg (470mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(11), Pg. 74, 1987. | |
| mouse | LD50 | subcutaneous | 630mg/kg (630mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: ANTIPSYCHOTIC VASCULAR: OTHER CHANGES | Toxicology and Applied Pharmacology. Vol. 4, Pg. 354, 1962. |
| rabbit | LD50 | oral | 450mg/kg (450mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Zdravookhranenie Turkmenistana. Public Health of Turkmenistan. Vol. 27(5), Pg. 9, 1983. |
| rabbit | LDLo | subcutaneous | 500mg/kg (500mg/kg) | Zeitschrift fuer Experimentelle Pathologie und Therapie. Vol. 1, Pg. 446, 1905. | |
| rat | LC50 | inhalation | 165ppm/7H (165ppm) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Journal of Toxicology and Environmental Health. Vol. 8, Pg. 59, 1981. |
| rat | LD50 | oral | 355mg/kg (355mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Zdravookhranenie Turkmenistana. Public Health of Turkmenistan. Vol. 27(5), Pg. 9, 1983. |
| rat | LD50 | skin | 1184mg/kg (1184mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Zdravookhranenie Turkmenistana. Public Health of Turkmenistan. Vol. 27(5), Pg. 9, 1983. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View